Odovtos-International Journal of Dental Sciences (Odovtos-Int. J. Dent. Sc.), Online First, 2025. ISSN: 2215-3411
https://doi.org/10.15517/kvtbk628

https://revistas.ucr.ac.cr/index.php/Odontos
CLINICAL RESEARCH:
Assessment of Serum Ferritin Levels and Periodontal Indices in Patients with
Chronic Periodontitis Post/Pre Radiation Treatment of Head and Neck Cancer
Evaluación de los niveles séricos de ferritina e índices periodontales en pacientes con periodontitis crónica antes y después del tratamiento con radioterapia por cáncer de cabeza y cuello
Ahmed A. Al-Kubaisi¹ https://orcid.org/0009-0002-6874-6346
Abdulrahman Khalid Mssdf² https://orcid.org/0009-0005-8859-8477
Sameer A. Awad¹ https://orcid.org/0000-0002-9194-719X
Khalid H. Ibrahim3 https://orcid.org/0009-0007-9872-1242
Mahmood Yaseen Mukhlif¹ https://orcid.org/0009-0004-9254-2380
Ekram R. Aldelaimi⁴ https://orcid.org/0009-0009-5890-6052
¹Department of Medical Laboratories Techniques, College of Health and Medical Technology, University of Al Maarif, Al Anbar, Iraq.
²Department of Clinical and Laboratory Science, College of Pharmacy, University of Anbar, Ramadi, Iraq.
3Abu Ghraib Hospital, Al-Karkh Health Directorate, Ministry of Health, Iraq.
⁴Department of Periodontology, Al-Anbar Health Directorate, Ministry of Health, Iraq.
Correspondence to: Ahmed A. Al-Kubaisi - ahmed.amer@uoa.edu.iq
Sameer A. Awad - sameer.msc1981@gmail.com
Received: 8-III-2025 Accepted: 4-VII-2025
ABSTRACT: Acute phase protein (APP) positivity is exhibited by ferritin in the presence of inflammation and chronic infections, such as periodontal disease. This study aims to investigate serum ferritin levels compared between post- and pre-radiation of periodontal disease with head and neck cancer PD+HNC. A total of 100 individuals in the present study were enrolled, comprising 50 patient’s periodontal disease (PD) with head and neck cancer who underwent radiotherapy post-six months (post-RT HNC) and 50 PD patients (pre-RT HNC). Probing pocket depth (PPD), clinical attachment level (CAL), gingival bleeding index (GBI), plaque index (PI), oral pH, and hyposalivation were noted. Serum ferritin levels were measured using an electrochemiluminescence immunoassay (eCLIA). A significantly higher percentage of ferritin levels was found in patients post-RT PD+HNC (301.90 ng/ml) contrasted to pre-RT PD+HNC (82.71 ng/ml) subjects. Serum ferritin levels were shown to be significantly positively correlated with CAL, PPD, PI, and GBI in subjects with chronic periodontitis. Receiver operating characteristic results of ferritin (area under the curve (AUC) =0.806 for post-RT PD+HNC, AUC=0.454 for pre-RT PD+HNC). It may be suggested that elevated serum ferritin levels in patients with post-RT PD+HNC have the potential to be evaluated as biomarkers for inflammation during periodontal tissue breakdown via radiation treatment.
KEYWORDS: Head and neck cancer; Periodontal disease; Ferritin; Radiotherapy.
RESUMEN: La ferritina, como proteína de fase aguda (APP), muestra positividad en presencia de inflamación e infecciones crónicas, como la enfermedad periodontal. Este estudio tiene como objetivo investigar los niveles séricos de ferritina en pacientes con enfermedad periodontal comparando entre el período posterior y previo a la radioterapia en cáncer de cabeza y cuello (PD+HNC). En total se incluyeron 100 individuos en el presente estudio: 50 pacientes con enfermedad periodontal y cáncer de cabeza y cuello que recibieron radioterapia después de seis meses (post-RT HNC) y 50 pacientes con enfermedad periodontal (pre-RT HNC). Se registraron la profundidad de sondaje periodontal (PPD), el nivel de inserción clínica (CAL), el índice de sangrado gingival (GBI), el índice de placa (PI), el pH oral y la hiposalivación. Los niveles séricos de ferritina se midieron mediante inmunoensayo por electroquimioluminiscencia (eCLIA). Se encontró un porcentaje significativamente mayor de niveles de ferritina en pacientes post-RT PD+HNC (301,90 ng/ml) en comparación con los sujetos pre-RT PD+HNC (82,71 ng/ml). Los niveles séricos de ferritina mostraron una correlación positiva significativa con CAL, PPD, PI y GBI en sujetos con periodontitis crónica. Los resultados de la curva característica operativa del receptor (ROC) para la ferritina mostraron un área bajo la curva (AUC) = 0,806 en post-RT PD+HNC y AUC = 0,454 en pre-RT PD+HNC. Se puede sugerir que los niveles elevados de ferritina sérica en pacientes post-RT PD+HNC tienen el potencial de ser evaluados como biomarcadores de inflamación durante la destrucción del tejido periodontal inducida por la radioterapia.
PALABRAS CLAVE: Cáncer de cabeza y cuello; Enfermedad periodontal; Ferritina; Radioterapia.
Introduction
Head and neck cancers (HNCs) are a broad category of malignant tumors that can arise in several anatomical locations, including the oral cavity, larynx, pharynx, oropharynx, nasopharynx, and sinonasal cavities. In 2018, 890,000 new cases of HNC and 450,000 recorded deaths, making it the seventh most common cancer worldwide (1). In 2023, about 2.4% of all cancer deaths in the US (14,500 deaths) and 4% of all cancer diagnoses (65,630 new cases) are related to HNC (2). Since symptoms typically arise at the final stage of the disease, late diagnosis is one of the main factors contributing to the high HNC mortality. Therefore, early identification and treatment are crucial to increase the likelihood of these cancers being cured and prevent major, mutilating procedures.
Periodontal disease, an inflammatory illness of the tooth-supporting structures marked by the gradual loss of periodontal tissues, is caused by an increase of anaerobic gram-negative or positive bacteria under the gum tissue line cause of periodontitis is the accumulation of bacterial plaque (biofilm) on teeth, if not removed, can lead to inflammation and destruction of the tissues supporting the teeth (3). Worldwide, periodontitis is a prevalent condition that poses a serious threat to public health in many nations (4, 5). In recent years, clinical markers such as probing depth (PD), clinical attachment level (CAL), bleeding on probing (BOP), and radiographic findings have been used to diagnose periodontitis (6). Rather than displaying the disease's present activity, these metrics frequently show prior periodontal disease. As a result, new diagnostic tests are needed to determine if the disease is present, how it will progress in the future, and how effectively the patient is responding to periodontal therapy, even when patients with periodontitis are already seeing clinical improvements (7).
Radiotherapy is the main treatment for the majority of HNC patients. It can be used alone or in combination with other therapies to destroy tumor cells. In addition to directly killing tumor cells, radiation therapy can also significantly affect the type and quantity of immune cells that invade the tumor and form the tumor-surrounding immunological microenvironment (8). HNC radiation results in a number of detrimental side effects, including a decrease in the immune system of the periodontium and a higher risk of periodontitis and attachment loss (9, 10). Furthermore, a patient's psychological, social, functional, and physical facets of their quality of life may be negatively impacted by periodontitis (11). For this reason, dental and medical professionals must keep a check on the oral hygiene of HNC patients undergoing radiation therapy (12). Therefore, an urgent need to identify sensitive biomarkers to enhance early diagnosis of tissue loss of the periodontium as a side effect of radiation. Many cells, such as immune cells, fibroblasts, keratinocytes, adipose tissue, and muscles, release pro-inflammatory cytokines (13), which can be a diagnostic biomarker for diagnostics, however, they are entrapped at the interface of bed and bench.
Current studies on the diagnosis of periodontal disease concentrate on the use of objective measurements, like as biomarkers, to determine the risk of periodontitis. APP biomarkers are among the molecules that can be used to track an individual's state of health, the start of a disease, how well a treatment is working, and how well the treatment is working (14). Alterations in the concentration of APPs are physiological changes brought on by tissue injury and inflammation. Numerous conditions, such as infection, trauma, infarction, inflammation, and different neoplasms, have been linked to APPs (15). In this case, APP known as ferritin rises in response to persistent infections, autoimmune diseases, liver diseases, and inflammation. Many chronic inflammations linked to diseases like adult-onset Still's disease, multiple sclerosis, and rheumatoid arthritis have been linked to elevated ferritin serum levels (16). Ferritin is a protein that stores iron within cells which binds with iron and releases it under specified conditions (17). Ferritin has an essential function in immune responses through the increasing movement of it from plasma to the cells. The results of that are bind iron to the host tissues yielding the defense mechanism against pathogens (18). Typically, extracellular fluids also contain ferritins. In certain medical situations, such as cancer, chronic infection, hepatocellular injury, and acute inflammation, elevated levels of ferritin can be observed in the bloodstream (19).
To our knowledge, there is no study in any cancer center in Iraq that investigated the levels of serum ferritin in periodontal disease (PD) who had either post-RT HNC or pre-RT HNC, as known that the levels of ferritin are increased in head and neck cancer who treated with radiotherapy as a potential to be evaluated as a biomarker for inflammation during periodontal tissue breakdown via radiation treatment. This study aimed to evaluate the levels of serum ferritin in individuals with periodontal disease (PD) and head and neck cancer (HNC), as compared between post- and pre-radiotherapy subjects.
Materials and Methods
Study Design and Population
We performed a prospective observational cross-sectional study conducted at the Cancer and Tumors Center/Anbar Cancer Centre (ACC), Iraq from September 2022 to January 2024. This study included 100 participants who visited ACC, (n=50) patients for each group (post-RT HNC and pre-RT HNC) with periodontal disease (PD). The individuals (n=50) patients post-RT PD+HNC were chosen from among those who underwent radiation treatment and additional systemic therapy. While (n=50) patients pre-RT PD+HNC were selected and treated with systemic therapy only. The National Comprehensive Cancer Network (NCCN) states that an oncologist at the Cancer and Tumors Centre made the HNC diagnosis for the participants (20). Al-Anbar of the Health Directorate, Ministry of Health, Iraq; authorized this study protocol (permission number: 2022057, August. 2. 2022). Informed consent was gained from all participants for their participation and the publication of the study results. Periodontal and common oral complications were examined by an experienced oral hygienist. To ensure that a comparable percentage of cases fit into the categories determined by the selection variable (age and sex), groups were chosen from an established age range (28-62) and sex. Males made up about 80% of the cases and controls.
Exclusion and Inclusion criteria
Individuals who met one of the following criteria were excluded: (a) either they had a history of salivary gland or oral diseases in the past, (b) they had a confirmed diagnosis of systemic disease, multiple sclerosis, or xerostomia, (c) a patient who declined to take part within the research. Moreover, those who met any of the preceding criteria were utilised for enrolling patients: (a) a malignant tumor of HNC was identified pathologically (derived from epithelial cells), (b) they have not had radiation treatment in the past, (c) There is no history of prior parotid, submandibular, or lingual salivary gland surgery, (d) there are no distant metastases, (e) a projected survival time of more than a year and overall physical state at a (0-1) score for performance, which is regarded as satisfactory.
Determination of ferritin levels
Venous blood was drawn from the antecubital vein into serum gel tubes and sera were extracted by centrifugation (10 min at 3500 rpm at 5°C). Modern technique an electrochemiluminescence immunoassay approach (eCLIA; FerritinTM test kit, Nipigon Health Corp, Ontario, Canada) was used to measure the serum levels of ferritin. The evaluation was performed according to the manufacturer's guidelines.
Physical examination of
periodontal indices
An experienced dentist by (E.R) performed periodontal indices post/pre-radiation. Periodontal parameters including plaque index, gingival bleeding index, clinical attachment level, and probing pocket depth were evaluated (21). Clinical characteristics were obtained in the third molars for all of the present teeth. Data on periodontal indices was obtained at four different locations per tooth and six places overall. From the point where the cement and enamel meet to the base of the gingival pockets, CAL was measured and scored. Based on whether there was blood loss for ten seconds following probing, the GBI was calculated (0 or 1, respectively) (22). A (0-3) point system was used to measure the PI; 0 meant no plaque. 1=distinct plaque particles near the tooth's cervical edge. 2=a thin, continuous band of plaque at the tooth's cervical edge, up to 1 mm in width. 3=a plaque band that is more than 1 mm in width but covers less than one-third of the tooth's crown (23). A millimeter periodontal probe was used for all hand probing measures. To diagnose patients, the updated Tonetti et al. (2018) periodontitis categorization system was used (24).
Statistical analysis
Categorical variables were characterized by frequency and percentage, and they were compared using the chi-square test. The mean±SD of data continuously distributed and normally were compared using an independent-sample t-test. The interquartile range and mean±SD for these variables were supplied. The correlation analysis was conducted using the Pearson correlation coefficient. To evaluate the efficacy of serum markers for both post-RT PD+HNC and pre-RT PD+HNC, the (AUC) of (ROC) curve analysis was employed. When p was less than 0.01 the differences were deemed statistically significant. IBM SPSS (v. 29, NY, USA) and GraphPad Prism (v. 10.2.3, La Jolla, CA, USA) were used to process all of the analyses.
Results
Population of study
The characteristics and population of all groups for this study are presented in Table 1. In this research population, there was a distribution of HNCs by gender and tumors sites have been shown in Figure 1. Many post-RT PD+HNC patients had a history of smoking (10 cigarettes per day, for more than 5 years), and the patients' mean age 40 ranged from 28 to 62 years of them, or 80%, were males. There were no patients in stage I, while stage II periodontitis affected a total of 9 (18%), stage III periodontitis affected 19 (38%), and stage IV periodontitis affected 22 (44%)/ grade C (n=50, 100%) in patients post-RT PD+HNC. The overall radiation exposure ranged from 5700 to 7000 cGy.
In this context, 50 of the post-RT PD+HNC patients (100%) received radiation treatment and combined systemic therapy (two to three doses) of cetuximab or cisplatin. While, 50 of pre-RT PD+HNC patients (100%) concurrently, received two to three doses of cetuximab or cisplatin as systemic treatment. In this research cohort, pre-RT PD+HNC patients with ages ranging from 28 to 60 on average, 40 (80%) of them were males. Thirty-one (62%) and five (10) of them had abused smoking and alcohol, respectively, and over half of individuals reported a history of smoking. The patients pre-RT PD+HNC were affected by stages I, II, III, and IV of periodontitis which were 15 (30%), 24 (48%), nine (18%), and two (4%)/ grade A (n=19, 38%) and B (n=31,62%), respectively.
Clinical periodontal markers, Oral saliva pH, and Hyposalivation
A significant statistical difference (p=0.001) was shown between the (post-RT PD+HNC) and (pre-RT PD+HNC) groups in terms of the increased CAL, PPD, PI, and GBI in post-RT PD+HNC as shown in Table 2. The mean values of oral saliva pH of the two groups (post-RT PD+HNC and pre-RT PD+HNC) varied, were 6.0±0.67 and 7.77±0.28, respectively, p=0.001. In addition, the average hyposalivation rates were about 0.15 and 0.30 millilitres per minute, respectively as seen in Table 2. Hyposalivation levels were considerably lower in the chronic periodontitis groups (p=0.001) in groups (post-RT PD+HNC) compared to (pre-RT PD+HNC) group.
Clinical activity and serum ferritin levels are strongly linked
Serum ferritin expression was elevated in a group with post-RT PD+HNC (301.90 [12.47–888.80] ng/mL) compared to the pre-RT PD+HNC group (82.71 [4.77–217.50] ng/ mL (p=0.001), as shown in Figure 2.
Serum ferritin was shown to be a more reliable marker of active patients with post-RT PD+HNC (area under the curve (AUC) = 0.806) compared to pre-RT PD+HNC (AUC = 0.454) in ROC analyses (Figure 3 and Table 3). Post-RT PD+HNC patients have higher ferritin levels (187.72 ng/ml), with a sensitivity of 60% and specificity of 98% for identifying clinical remission (p=0.001). Ferritin levels were lowered at about (133.25 ng/ml) in patients which having pre-RT PD+HNC, and these levels could detect clinical remission and had a sensitivity of 40% with a specificity of 86% (p=0.432).
Serum Ferritin Levels and Correlation with Periodontal indecs, Oral saliva pH, and Hyposalivation
A Pearson correlation coefficient was identified, and the clinical periodontal measures scores; PI, GBI, PPD, and CAL, showed a positive statistically significant with serum ferritin level (p<0.001). Additionally, there was a negative statistically significant association between the serum ferritin level and the pH of oral saliva and hyposalivation, as seen in Table 4.
Table 1. The characteristics and population of cases.
|
Variables |
Post-RT PD+HNC |
Pre-RT PD+HNC |
p |
|
Age (years), Mean±SD |
41.34±8.41 |
40.06±6.41 |
n.s |
|
BMI, Mean±SD |
26.01±5.74 |
27.91± 4.61 |
0.53 |
|
Stage of Tumor, n (%) |
|||
|
1-2 |
9(18) |
40(80) |
0.001* |
|
3-4 |
41(82) |
10(20) |
<0.001* |
|
Smoking, n (%) |
|||
|
Yes |
72)36) |
31 (62) |
0.001* |
|
No |
14 (28) |
19 (38) |
0.001* |
|
Alcohol Drinking, n (%) |
|||
|
Yes |
13 (26) |
5 (10) |
0.001* |
|
No |
37 (74) |
45 (90) |
0.001* |
|
Type of treatment, n (%) |
|||
|
Chemotherapy |
0 (0.0) |
50(100) |
- |
|
Chemoradiotherapy |
50 (100) |
0 (0.0) |
- |
PD: Periodontal disease; Post-RT HNC: Post-radiotherapy Head and Neck Cancer, Pre-RT HNC: Pre-radiotherapy Head and Neck Cancer, SD: Standard Deviation, RT: Radiotherapy.
n.s: non-significant (p>0.01)
*: Statistically significant at (p<0.01)

Figure 1. Distribution of HNCs study involved: (A) HNCs patients by gender and demographical data. (B) tumor sites.
Table 2. Clinical characteristics of head and neck cancer post/pre-radiation treatment of periodontal health.
|
Variables |
Post-RT PD+HNC |
Pre-RT PD+HNC |
p |
|
CAL (mm) |
7.02 ± 0.42 |
6.34 ± 0.77 |
0.001* |
|
PPD (mm) |
8.1 ± 0.46 |
7.12 ± 0.61 |
0.001* |
|
PI (mm) |
2.52 ± 0.61 |
1.94 ± 1.03 |
0.001* |
|
GBI (%) |
90.37 ± 0.57 |
63.13± 0.61 |
0.001* |
|
Oral saliva pH |
6.0±0.67 |
7.77±0.28 |
0.001* |
|
Hyposalivation (ml/min) |
0.15±0.04 |
0.30±0.04 |
0.001* |
Note: values are expressed as mean±SD
*: Statistically significant at p<0.01
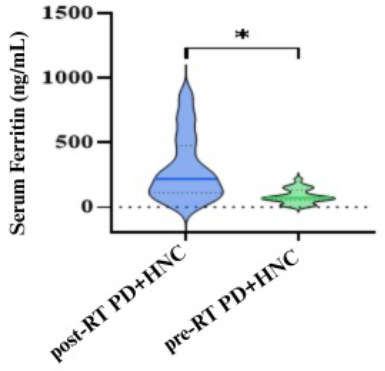
Figure 2. Serum ferritin levels in post-RT PD+HNC and pre-RT PD+HNC individuals are plotted as a violin, showing the mean, interquartile range, and upper and lower values involved, (*: Significantly at p<0.01).
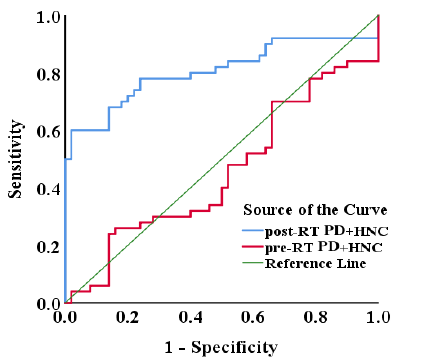
Figure 3. Receiver Operating Characteristic (ROC) Curve for Ferritin Prediction in Pre-RT and Post-RT PD+HNC Patients.
Table 3. ROC evaluations of ferritin levels in serum to determine clinical remission.
|
Variable |
Groups |
A.U.C. |
Cut-off (ng/ml) |
Sensitivity % |
Specificity % |
p |
|
Ferritin (ng/ml) |
Post-RT PD+ HNC |
0.806 |
187.72 |
60 |
98 |
0.001* |
|
Pre-RT PD+HNC |
0.454 |
133.25 |
24 |
86 |
0.432⁋ |
*: significant p<0.01
⁋: non-significant p>0.01
Table 4. Correlation of serum ferritin level with PPD, CAL, PPD, GBI, PI, Oral saliva pH, and Hyposalivation.
|
Variables |
Ferritin (ng/ml) |
|
|
r |
p |
|
|
Clinical attachment level |
0.318 |
0.001* |
|
Periodontal pocket depth |
0.379 |
0.001* |
|
Gingival bleeding index |
0.412 |
0.001* |
|
Plaque index |
0.239 |
0.001* |
|
Oral saliva pH |
-0.389 |
0.001* |
|
Hyposalivation |
-0.478 |
0.001* |
*: Significant p<0.01.
Discussion
The main objective of the present research was to investigate the relationship between clinical periodontal index activity and serum ferritin levels in the post-radiation and pre-radiation HNCs. Our results showed that increase in serum ferritin in patients post-RT PD+HNC. The serum ferritin levels within the normal range are between 12-145 ng/ml. Despite the significant effect of radiotherapy on HNC patients, serum ferritin levels remain within the normal range (82.71 ng/ml) in pre-RT PD+HNC. When periodontal tissues are affected by periodontitis, ferritin expression is markedly positive. In this line, P. gingivalis gives rise to elevated levels of LPSc, IL-6, suPAR and TNF-α in patients of periodontitis which causes a rise in the expression and secretion of ferritin (25, 26). Extracellular ferritin stimulates human periodontal ligament cells to produce pro-inflammatory cytokines, which may amplify the immune-inflammatory reactions associated with periodontitis (25, 27). When combined, ferritin may have a role in the onset of periodontitis (28). Previous studies have revealed that ferritin was extensively dispersed and constitutively present in the periodontal tissues of primates, where it may be involved in angiogenesis, mineralization, inflammation, and epithelial proliferation (29). Additionally, a study by Faramarzi et al. found that inflammation may increase ferritin expression in the blood of individuals with chronic periodontitis. Bacterial load and virulence factors of the bacteria are other variables that may potentially contribute to elevated serum ferritin levels (30). In order investigate the periodontium-damaging effects of HNC radiation, the current study primarily focused on periodontal indices and saliva pH alterations.
In patients post-RT PD+HNC as compared to pre-RT PD+HNC, oral saliva pH was reduced (acidic) to almost alkaline. These findings were in agreement with previous studies demonstrating that those who received radiation have rather acidic saliva because radiation damage reduces the buffering capacity of the body (31) mostly on the salivary glands' serous acini (32). Periodontitis may be more likely as a result of hyposalivation and the diminished preventive benefits of saliva on oral health. According to results obtained, patients' hyposalivation was reduced after RT PD+HNC which was more before RT PD+HNC. Background information is given, and it is examined here if periodontal indices and hyposalivation may be utilised to concern and describe potential HNC radiation-induced deterioration of oral and periodontal tissues. For several reasons, HNC patients who receive radiotherapy have a higher chance of developing periodontal diseases than those in the general population (33). Following the initiation of radiotherapy, poorer periodontal health will occur dose-dependently (34). Furthermore, radiation therapy to the head and neck region alters the oral microbiome, which leads to dysbiosis caused by bacteria associated with periodontal disease (35). This single-variate analysis found a significant negative correlation between hyposalivation and periodontal indices with the occurrence of periodontitis. This is consistent with the findings of our investigation. The increased periodontal loss observed in post-RT PD+HNC patients may indicate a clear relationship between periodontal characteristics and hyposalivation.
The sample size, the study's shortcomings include its brief duration and inability to exclude out some confounding factors, such the use of chemotherapy. Finally, a substantial amount of research indicates that post-RT HNC, periodontal disease, and inflammatory factors are all related to one another. Although there is a potential causal relationship between chronic periodontitis and both pre-RT and post-RT HNC, data on the diagnostic utility of biomarkers remain unavailable. This is caused by the wide range of differences in the study's design, people involved, assay methods, and biomarker analysis. Future randomized control and prospective studies with standardized clinical and biological data are required to demonstrate the causal association between post-RT HNC and periodontitis. Additionally, additional research is needed to determine the importance of biomarkers in linking these disorders together.
Conclusions
The findings of this study are significant in terms of using ferritin as a biomarker to assess the severity of periodontitis, especially in patients post/pre-radiotherapy. Thus, these results also suggested that iron overload in PD with post-RT HNC patients might have the potential to be evaluated as a biomarker for inflammation during periodontal tissue breakdown via radiation treatment. Furthermore, post-RT patients exhibited more severe periodontitis (stages III-IV, grade C), while pre-RT patients were mostly in earlier stages (I-II, grades A-B). All patients received radiation and systemic therapy (cetuximab or cisplatin). These findings suggest a strong association between treatment stage, lifestyle habits, and periodontitis severity in HNC patients.
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study or from their caregivers.
Conflicts of Interest: The authors declare no conflict of interest.
AUTHOR CONTRIBUTION STATEMENT: Conceptualization: S.A.A. and A.A.A.K; Methodology: A.K.M, K.H.I. and M.Y.M.; Formal analysis: E.R.A. and A.K.M.; Writing-original draft preparation: A.K.M., M.Y.M. and A.A.A.K.; Writing-review and editing: A.A.A.K. and S.A.A; Supervision: A.A.A.K. and S.A.A. All authors have read and agreed to the published version of the manuscript.
Funding: This research received no external funding.
References
1. Statistics, E.G.C., GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 2020. 70 (4): p.313.
2. Siegel, R.L., et al., Cancer statistics, 2023. CA: a cancer journal for clinicians, 2023. 73 (1): p.17-48.
3. Saliem, S.S., et al., Pathogenesis of periodontitis-A potential role for epithelial-mesenchymal transition. Japanese Dental Science Review, 2022. 58: p.268-278.
4. Bertolini, M. and D. Clark, Periodontal disease as a model to study chronic inflammation in aging. Geroscience, 2024. 46 (4): p.3695-3709.
5. Albukhalefah, A.K.M. and H.A.N. Nayyef, Association between biochemical lepetin and some biochemical markers in Iraqi patients with obesity and periodontal. Romanian Journal of Diabetes, Nutrition and Metabolic Diseases, 2024. 31 (1): p.569-586.
6. Kwon, T., I.B. Lamster, and L. Levin, Current concepts in the management of periodontitis. International dental journal, 2021. 71 (6): p.462-476.
7. Gajjar, S., et al., Effect of serum ferritin levels in gingivitis and periodontitis patients before and after nonsurgical periodontal therapeutic intervention. Journal of Applied Pharmaceutical Science, 2023. 13 (11): p.152-160.
8. Karam, S.D. and D. Raben, Radioimmunotherapy for the treatment of head and neck cancer. The Lancet Oncology, 2019. 20 (8): p.e404-e416.
9. Kutuk, T., et al., Interdisciplinary Collaboration in Head and Neck Cancer Care: Optimizing Oral Health Management for Patients Undergoing Radiation Therapy. Current Oncology, 2024. 31 (4): p.2092-2108.
10. Moshtaha, W., Oral Complications of Dental Prosthetic for Patients after Chemotherapy and Radiotherapy Treatment. Dental Hypotheses, 2021. 12 (2): p.67-72.
11. Costa, A.A., et al., The association between periodontitis and the impact of oral health on the quality of life of individuals with psoriasis and psoriatic arthritis. PloS one, 2024. 19 (6): p.e0301158.
12. Irie, M.-S., et al., Periodontal therapy for patients before and after radiotherapy: A review of the literature and topics of interest for clinicians. Medicina oral, patologia oral y cirugia bucal, 2018. 23 (5): p.e524.
13. Pal, P.P., et al., New natural pro-inflammatory cytokines (TNF-α, IL-6 and IL-1β) and iNOS inhibitors identified from Penicillium polonicum through in vitro and in vivo studies. International Immunopharmacology, 2023. 117: p.109940.
14. Zia, A., et al., Oral biomarkers in the diagnosis and progression of periodontal diseases. Biology and Medicine, 2011. 3 (2): p.45-52.
15. Powanda, M.C. and E.D. Moyer, A brief, highly selective history of acute phase proteins as indicators of infection, inflammation and injury. Inflammopharmacology, 2021. 29 (3): p.897-901.
16. Uppal, S.S., et al., Ten years of clinical experience with adult onset Still’s disease: is the outcome improving? Clinical rheumatology, 2007. 26: p.1055-1060.
17. Sudarev, V.V., et al., Ferritin self-assembly, structure, function, and biotechnological applications. International journal of biological macromolecules, 2023. 224: p.319-343.
18. Chow, J.K., et al., Increased serum iron levels and infectious complications after liver transplantation. Clinical Infectious Diseases, 2010. 51 (3): p.e16-e23.
19. Sandnes, M., et al., Hyperferritinemia-a clinical overview. Journal of Clinical Medicine, 2021. 10 (9): p.2008.
20. Caudell, J.J., et al., Head and neck cancers, version 1.2022 featured updates to the NCCN guidelines. JNCCN Journal of the National Comprehensive Cancer Network, 2022. 20 (3): p.225-234.
21. Mohd Noh, N.Z. and E. Noor, Gingival recession at a glance. BDJ Student, 2024. 31 (2): p.64-65.
22. Barnes, C.M., et al., Comparison of irrigation to floss as an adjunct to tooth brushing: effect on bleeding, gingivitis, and supragingival plaque. Journal of Clinical Dentistry, 2005. 16 (3): p.71.
23. Gil-Montoya, J.A., et al., Oral and general health conditions involved in periodontal status during pregnancy: A prospective cohort study. Archives of Gynecology and Obstetrics, 2023. 308 (6): p.1765-1773.
24. Tonetti, M.S., H. Greenwell, and K.S. Kornman, Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. Journal of periodontology, 2018. 89: p.S159-S172.
25. Ahmad Akhoundi, M., et al., Urokinase-plasminogen activator protects periodontal ligament fibroblast from oxidative induced-apoptosis and DNA damage. Journal of Periodontal Research, 2018. 53 (5): p.861-869.
26. Al-Kubaisi, A.A., et al., Soluble urokinase plasminogen activator receptor (suPAR) is a potential biomarker of stage III-IV, grade C periodontitis through the impact of post-radiotherapy on head and neck cancer patients. BMC Oral Health, 2024. 24 (1): p.1144.
27. Al-Kubaisi, A.A., et al., Changes and Roles of IL-6, hsCRP, and proCT in Patients with Chronic Periodontitis in Head and Neck Cancer Pre/Post Radiotherapy. Al-Rafidain Journal of Medical Sciences (ISSN 2789-3219), 2024. 7 (1): p.248-254.
28. Huang, W., et al., Up-regulated ferritin in periodontitis promotes inflammatory cytokine expression in human periodontal ligament cells through transferrin receptor via ERK/P38 MAPK pathways. Clinical Science, 2019. 133 (1): p.135-148.
29. Huang, W., et al., Ferritin expression in the periodontal tissues of primates. European Journal of Histochemistry: EJH, 2019. 63 (3).
30. Faramarzi, M., et al., The effect of adjunctive use of melatonin as a supplement on serum ferritin level in periodontal patients: A randomized, controlled trial. Dental Research Journal, 2021. 18 (1): p.39.
31. Tiwana, M., et al., Whole saliva physico-biochemical changes and quality of life in head and neck cancer patients following conventional radiation therapy: a prospective longitudinal study. Indian journal of cancer, 2011. 48 (3): p.289-295.
32. Pontes, C.d.B., A.C.M. Polizello, and A.C.C. Spadaro, Clinical and biochemical evaluation of the saliva of patients with xerostomia induced by radiotherapy. Brazilian oral research, 2004. 18: p.69-74.
33. Sroussi, H.Y., et al., Common oral complications of head and neck cancer radiation therapy: mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer medicine, 2017. 6 (12): p.2918-2931.
34. Hommez, G.M., et al., Effect of radiation dose on the prevalence of apical periodontitis-a dosimetric analysis. Clinical oral investigations, 2012. 16: p.1543-1547.
35. Laheij, A.M. and J.J. de Soet, Can the oral microflora affect oral ulcerative mucositis? Current opinion in supportive and palliative care, 2014. 8 (2): p.180-187.
 Odovtos -Int J Dent Sc endoses to CC-BY-NC-SA 4.0.
Odovtos -Int J Dent Sc endoses to CC-BY-NC-SA 4.0.