Odovtos-International Journal of Dental Sciences (Odovtos-Int. J. Dent. Sc.), Online First, 2025. ISSN: 2215-3411
https://doi.org/10.15517/vg7z8h38

https://revistas.ucr.ac.cr/index.php/Odontos
LETTERS TO THE EDITOR:
Nanoleakage in Dentistry: 30 Years Later
Nanofiltración en odontología: 30 años después
José Luis Álvarez-Vásquez DDS¹ https://orcid.org/0000-0003-0381-2402
Nicholas G. Fischer DDS, PhD² https://orcid.org/0000-0003-2230-5158
¹Full Professor, Discipline of Endodontics, Faculty of Dentistry, University of Cuenca, Ecuador.
²Fellow, Department of Bioengineering, University of Pennsylvania, Philadelphia, Pennsylvania, USA. Center for Precision Engineering for Health, University of Pennsylvania, Philadelphia, Pennsylvania, USA. Center for Innovation and Precision Dentistry, University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Correspondence to: Dr. José Luis Álvarez-Vásquez - jose.alvarezv@ucuenca.edu.ec
Received: 19-VIII-2025 Accepted: 19-IX-2025
"The real voyage of discovery consists not in seeking new landscapes, but in having new eyes"
Marcel Proust
Dear Editor,
This letter addresses the article recently published by Medina et al. (1). In their in vitro study, the authors evaluated the marginal sealing of a bulk-fill nanocomposite. They found that marginal sealing varies by curing protocols and aging processes, evidencing the need for optimizing curing protocols to provide better long-term durability of composite restorations. Notably, they used microleakage and thermocycling; the former was measured with dye penetration. The authors clearly point out the limitations of their study (1), however it is salient to recognize that, although dye penetration is a very popular method used by many authors, several weaknesses have been reported for this model by prominent dental journals (2) and authors in this Journal (Brenes-Valverde et al., for example) (3). In their manuscript, Medina et al. state that “with progress in nanotechnologies and an increasing availability of nanocomposites….”, new classes of composites are emerging (1). In this regard, we believe the nanoleakage model would have been more appropriate, as it better corresponds to the nanoleakage scale.
Indeed, it has been 30 years since the publication of Sano et al.’s seminal articles on nanoleakage in 1995 (4,5). These groundbreaking studies opened our eyes to the fact that, even in the presence of gap-free restorations and sound clinical practice, leakage occurs through submicron spaces (20-100 nm) within incompletely infiltrated hybrid layers. Thus, adhesive systems cannot completely prevent the passage of fluids across these defective hybridization zones (4,5). This persistent water uptake weakens the adhesive interface and ultimately drives restoration failure.
Importantly, “water is the nemesis of the longevity of the hybrid layer” (7). Water sorption has been identified as one of the primary causes of nanoleakage, leading to hydrolytic degradation of resin-dentin bonds and the progressive widening of nanoleakage pathways (8). The formation of the so-called “water-tree” structures can occur in as little as 30 minutes, beginning immediately after the bonding procedure (9,10). Hydrolysis is further compounded by the biodegradation of resin composites and adhesive systems (11,12) and the inherent bacterial leakage, daily temperature fluctuations in the oral environment (13), and the role of microbial adhesion on biomaterials (14). Additionally, both thermal and mechanical stresses significantly increase nanoleakage (15).
Despite the passage of three decades since the term 'nanoleakage' was introduced, research in this area remains sparse, with a PubMed search for 'nanoleakage' returning only 490 publications to date (16) (Figure 1). The conventional nanoleakage detection methods - scanning electron microscopy (SEM) and transmission electron microscopy (TEM) with silver nitrate - are still widely used, despite their inherent limitation of providing only two-dimensional information. However, TEM has enabled the identification of distinct water - induced nanoleakage patterns correlating to different bonding strategies, including the aforementioned water-tree structures (8). More advanced techniques such as dual-beam systems (focused ion beam/SEM) and electron tomography have been successfully applied to quantify nanoleakage in 3D at high resolution, avoiding potential artifacts associated with SEM and TEM (17).
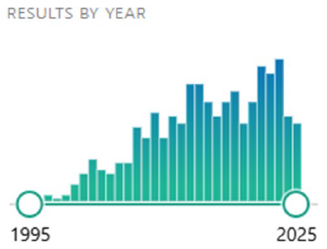
Figure 1. Timeline of 490 total publications pertaining to “nanoleakage” in PubMed, from inception of the term to 2025. Source: PubMed database.
Notably, despite significant advances in adhesive dentistry over recent decades (7,18), nanoleakage has been demonstrated even with new-generation adhesive systems (4,18). Today, clinicians have a wide array of adhesive systems available for restorative and prosthodontic cementation purposes. Furthermore, there is constant development and release of restorative materials with alleged improved mechanical and esthetic properties, yet many have not been evaluated from a nanoleakage perspective. Importantly, pulpal deterioration may occur after restorative procedures (19), but dental pulp responses to nanoleakage remain unexplored in the available literature.
While nanoleakage is far less extensive than microleakage (8), its true clinical implications remain uncertain. This should motivate researchers and academicians to design studies aimed at reducing or eliminating nanoleakage. Although some strategies, such as the use of natural products like fucosterol (20), immediate dentin sealing (21), or the incorporation of dendrimers into adhesives (22) have been proposed, much more work remains to be done since Sano et al.’s seminal publications. Future investigations should analyze failed restorations retrieved from patients to provide deeper insight into the mechanisms underlying clinical failure.
Several important questions still lack answers:
1. Are we, as clinicians and academicians, settling for competence rather than striving for excellence (23) when approaching nanoleakage reduction/elimination?
2. Is nanoleakage purely a material-related issue or should new clinical techniques be introduced?
3. Is the resin-bonding interface truly a “never-ending story” when approaching nanoleakage reduction/elimination (24)?
4. To what grade does nanoleakage impact the longevity of restorations?
Additionally, the data from in vitro studies, often showing improvements in adhesion, are still far from matching the clinical performance of restorations (18). Perhaps cutting-edge approaches, such as bioinspired microspheres to enhance dental adhesion (25) or the incorporation of bioactive molecules (18), could help address this complex problem.
Hopefully, this manuscript encourages us, including the authors of the study addressed in this letter, to approach nanoleakage with “new eyes,” because the “mission accomplished” in its reduction or elimination has not yet been truly achieved. Without question, further research is warranted as we strive to provide patients with long-standing restorations.
Author contribution statement: Conceptualization and design: J.L.A.V.; Literature review: J.L.A.V.; Methodology and validation: J.L.A.V.; Formal analysis: J.L.A.V. and N.G.F.; Data analysis and interpretation: J.L.A.V. and N.G.F.; Writing-original draft preparation: J.L.A.V.; Writing-review & editing: J.L.A.V. and N.G.F.; Supervision: J.L.A.V.; Project administration: J.L.A.V.”
References
1. Medina J., Quispe Tasayco L., Orellana Arauco H., Muñoz W., Espinoza Carhuancho F., Mayta Tovalino F. In vitro analysis of marginal sealing using light curing techniques on aged and unaged composite resins. Odovtos-International Journal of Dental Sciences. 2025: 111-120.
2. Editorial Board of the Journal of Endodontics. Wanted: a base of evidence. Journal of Endodontics. 2007; 33 (12): 1401-1402.
3. Brenes Valverde K., Conejo Rodríguez E., Vega Baudrit J.R., Montero Aguilar M., Chavarría Bolaños D. Evaluation of microleakage by gas permeability and marginal adaptation of MTA and Biodentine™ apical plugs: in vitro study. Odovtos-International Journal of Dental Sciences. 2018; 20 (1): 57 67.
4. Sano H., Takatsu T., Ciucchi B., Horner J.A., Matthews W.G., Pashley D.H. Nanoleakage: leakage within the hybrid layer. Oper Dent. 1995; 20 (1):18-25.
5. Sano H., Yoshiyama M., Ebisu S., et al. Comparative SEM and TEM observations of nanoleakage within the hybrid layer. Oper Dent. 1995; 20 (4): 160-167.
6. Maravic T., Mazzitelli C., Mayer-Santos E., et al. Current Trends for Cementation in Prosthodontics: Part 1-The Substrate. Polymers (Basel). 2025; 17 (5): 566.
7. Breschi L., Maravic T., Mazzitelli C., et al. The evolution of adhesive dentistry: From etch-and-rinse to universal bonding systems. Dent Mater. 2025; 41 (2): 141-158.
8. Hashimoto M., Yamaguchi S., Imazato S. Nanoleakage and Durability of Resin/Dentin Bonds. Curr Oral Health Rep. 2015, 2: 195-201.
9. Hashimoto M. et al. Diffusion-induced water movement within resin-dentin bonds during bonding. J Biomed Mater Res B Appl Biomater. 2006; 79 (2): 453-8.
10. Hashimoto M. et al. Permeability of adhesive resin films. J Biomed Mater Res B Appl Biomater. 2005; 74 (2): 699-705).
11. Mokeem L.S., Garcia I.M., Melo M.A. Degradation and Failure Phenomena at the Dentin Bonding Interface. Biomedicines. 2023; 11 (5): 1256.
12. Betancourt D.E., Baldion P.A., Castellanos J.E. Resin-Dentin Bonding Interface: Mechanisms of Degradation and Strategies for Stabilization of the Hybrid Layer. Int J Biomater. 2019; 2019: 5268342.
13. Ayatollahi M.R., Yahya M.Y., Karimzadeh A., Nikkhooyifar M., Ayob A. Effects of temperature change and beverage on mechanical and tribological properties of dental restorative composites. Mater Sci Eng C Mater Biol Appl. 2015; 54: 69-75.
14. Olivares A., Barraza V., Aguayo S. Micro- and nano-scale adhesion of oral bacteria to biomaterials using atomic force microscopy: A systematic review. Jpn Dent Sci Rev. 2025; 61: 41-54.
15. El-Keredy D., Etman W., Salama M. Nanoleakage of different composite restoration systems. Tanta Dental Journal; 2020; 17 (3): 97-105.
16. https://pubmed.ncbi.nlm.nih.gov/?term=nanoleakage. Accessed: August 01, 2025.
17. Coutinho E., Cardoso M.V., Fernandes C.P., et al. Nanoleakage distribution at adhesive-dentin interfaces in 3D. J Dent Res. 2011; 90 (8): 1019-1025.
18. Cadenaro M., Josic U., Maravić T., et al. Progress in Dental Adhesive Materials. J Dent Res. 2023; 102 (3): 254-262.
19. Desai S., Tepperman A., Ben Suleiman A., et al. Pulpal Deterioration Following Restorative Procedures: A Case-Control Study. J Endod. Published online June 13, 2025.
20. Kim H., Jung Y.J., Kim Y., et al. Long-term hybrid stability and matrix metalloproteinase inhibition by fucosterol in resin-dentin bonding biomechanics. Sci Rep. 2024; 14 (1): 20415.
21. Santana V.B., de Alexandre R.S., Rodrigues J.A., Ely C., Reis A.F. Effects of Immediate Dentin Sealing and Pulpal Pressure on Resin Cement Bond Strength and Nanoleakage. Oper Dent. 2016; 41 (2): 189-199.
22. Vasconcelos e Cruz J., Brito J., Polido M., Gonçalves L. A new experimental adhesive system containing G-IEMA-physicochemical properties. J Adhes Sci Technol. 2018; 33 (4): 418-432.
23. Diefenderfer K. Has Competence Replaced Excellence? A Reprint with Foreword. Oper Dent. 2024; 49 (1): 3-4.
24. Bertassoni L.E., Orgel J.P., Antipova O., Swain M.V. The dentin organic matrix - limitations of restorative dentistry hidden on the nanometer scale. Acta Biomater. 2012; 8 (7): 2419-2433.
25. Yao C., Liang S., Yu M., et al. High-Performance Bioinspired Microspheres for Boosting Dental Adhesion. Small. 2024; 20 (29): e2310251.
 Odovtos -Int J Dent Sc endoses to CC-BY-NC-SA 4.0.
Odovtos -Int J Dent Sc endoses to CC-BY-NC-SA 4.0.