Odovtos-International Journal of Dental Sciences (Odovtos-Int. J. Dent. Sc.), Online First, 2025. ISSN: 2215-3411
https://doi.org/10.15517/w37rx029

https://revistas.ucr.ac.cr/index.php/Odontos
BASIC RESEARCH:
Effect of Cetrimide with Bioceramic or Calcium Hydroxide Intracanal Medication
Efecto del cetrimida con medicamento intracanal de biocerámica o hidróxido de calcio
Larissa Braz Pontes DDS, MsC¹ https://orcid.org/0000-0001-7609-5059
Camila Soares Lopes DDS, PhD¹ https://orcid.org/0000-0002-7746-1580
Jéssica Arielli Pradelli DDS, PhD¹ https://orcid.org/0000-0003-3591-7364
Mário Tanomaru-Filho DDS, PhD¹ https://orcid.org/0000-0002-2574-4706
Juliane Maria Guerreiro-Tanomaru DDS, PhD¹ https://orcid.org/0000-0003-0446-2037
¹Department of Restorative Dentistry, Araraquara School of Dentistry, São Paulo State University-UNESP, Araraquara, SP, Brazil.
Correspondence to: PhD. Mário Tanomaru-Filho - tanomaru@uol.com.br
Received: 5-VIII-2025 Accepted: 15-VIII-2025
ABSTRACT: This study evaluated the pH, solubility, and intratubular decontamination of bioceramic intracanal medications Bio-C® Temp (BCT), a calcium hydroxide-based paste Calen® (CAL), and their associations with 1% Cetrimide (CTR): BCT, CAL, BCT/CTR, CAL/CTR. Polyethylene tubes filled with the medications were used for pH measurement at intervals of 1, 3, 7, 14, and 21 days. Solubility was evaluated after 14 days of immersion in distilled water at 37°C. Bovine dentin tubules contaminated with Enterococcus faecalis were used to assess intratubular decontamination using confocal laser scanning microscopy and Live/Dead BacLight Bacterial dye three days after exposure to the medications and a Control Group using Polyethylene glycol 400. Statistical analysis was performed using ANOVA and Tukey tests (p<0.05). CAL/CTR exhibited the highest pH after 1 day (p<0.05). CAL had the highest pH values at 14 and 21 days (p<0.05). BCT showed the lowest pH values at 3 days, and BCT/CTR at 7 and 21 days (p<0.05). The association of CTR did not affect the solubility of the medications (p > 0.05). CAL demonstrated superior intratubular decontamination (p<0.05), while CAL/CTR, BCT, and BCT/CTR showed similar results (p>0.05). The association of 1% cetrimide did not increase the intratubular decontamination of Bio-C® Temp and decreased the antibacterial activity of Calen®. The calcium hydroxide medication in a viscous vehicle exhibited higher alkaline potential, solubility, and intratubular decontamination.
KEYWORDS: Calcium hydroxide; Dental materials; Endodontics; Enterococcus faecalis; Root canal therapy; Silicates.
RESUMEN: Este estudio evaluó el pH, la solubilidad y la descontaminación intratubular de las medicaciones intracanal biocerámicas Bio-C® Temp (BCT), una pasta a base de hidróxido de calcio Calen® (CAL) y sus combinaciones con Cetrimida al 1% (CTR): BCT, CAL, BCT/CTR, CAL/CTR. Se utilizaron tubos de polietileno llenos con las medicaciones para medir el pH a intervalos de 1, 3, 7, 14 y 21 días. La solubilidad se evaluó después de 14 días de inmersión en agua destilada a 37°C. Se utilizaron túbulos dentinarios bovinos contaminados con Enterococcus faecalis para evaluar la descontaminación intratubular mediante microscopía confocal de escaneo láser y el tinte bacteriano Live/Dead BacLight, tres días después de la exposición a las medicaciones y a un grupo de control utilizando polietilenglicol 400. El análisis estadístico se realizó mediante pruebas ANOVA y Tukey (p<0.05). CAL/CTR mostró el pH más alto después de 1 día (p<0.05). CAL tuvo los valores de pH más altos a los 14 y 21 días (p<0.05). BCT mostró los valores de pH más bajos a los 3 días, y BCT/CTR a los 7 y 21 días (p<0.05). La adición de CTR no afectó la solubilidad de las medicaciones (p>0.05). CAL demostró una descontaminación intratubular superior (p<0.05), mientras que CAL/CTR, BCT y BCT/CTR mostraron resultados similares (p>0.05). La inclusión de cetrimida al 1% no mejoró la descontaminación intratubular de Bio-C® Temp y redujo la actividad antibacteriana de Calen®. Calen® en un vehículo viscoso exhibió un mayor potencial alcalino, solubilidad y descontaminación intratubular.
PALABRAS CLAVE: Hidróxido de calcio; Materiales dentales; Endodoncia; Enterococcus faecalis; Terapia de conducto radicular; Silicatos.
INTRODUCTION
The complexity of root canal systems (RCS) makes it difficult to achieve the complete elimination of microorganisms and could compromise the success of endodontic treatment (1). The aim of intracanal medication is to complement the disinfection of the RCS (2). Enterococcus faecalis is a gram-positive bacterium associated with cases of endodontic treatment failure, due to its capacity to survive under conditions of nutritional restriction, invade dentinal tubules and form biofilms (1).
Bio-C® Temp (BCT, Angelus, Londrina- PR, Brazil), is a ready-to-use paste proposed for use as a bioceramic intracanal medication in various endodontic procedures such as conventional treatment, retreatment, apexification, and regeneration (3). It is composed of calcium silicates, calcium aluminate, calcium oxide, resin base, calcium tungstate, and titanium oxide (3). Bio-C® Temp has an alkaline pH (3-6), radiopacity (4, 5, 7), releases calcium ions (4-6), is biocompatible (8), and has bioactive potential (9). Residues of BCT on the dentin wall have been shown to interact chemically with a bioceramic cement, Bio-C Sealer, forming a biomineralizing layer that enhances bond strength (10). However, long-term use of BCT may lead to coronary discoloration (11). Additionally, BCT exhibits lower antibacterial and antibiofilm activity against E. faecalis compared to calcium hydroxide medication (12-14).
Calcium Hydroxide (Ca(OH)₂) is widely used as an intracanal medication due to its excellent biological properties and antimicrobial activity (15). However, it may be less effective against resistant microorganisms like E. faecalis (2). Calen® (CAL, S. S. White Artigos Dentários Ltda, Rio de Janeiro-RJ, Brazil) is an intracanal medication based on Ca(OH)2 associated with a viscous vehicle, Polyethylene glycol 400. Associations with antimicrobial agents is suggested to potentiate the antimicrobial action of medications (16, 17).
Cetrimide (CTR) is a cationic surfactant that is effective against gram-positive and gram-negative bacteria (18-20). It also has residual antimicrobial action (21). This surfactant can help destroy bacterial biofilms by disrupting the cohesive forces between the polymeric matrix and the bacteria (22). Additionally, CTR reduces surface tension, favoring its penetration into hard-to-reach places, such as dentinal tubules and ramifications of root canals (23). When associated with endodontic materials such as irrigant solutions and endodontic sealer, CTR enhances antibacterial activity against E. faecalis (22, 24). Therefore, the addition of CTR to intracanal medications may increase antibacterial activity.
The aim of this study was to evaluate the impact of CTR on the physicochemical properties and intratubular decontamination of the association of cetrimide with bioceramic medications, BCT, which are based on Ca(OH)2, CAL®. The null hypothesis was that the association of CTR with CAL and BCT medications would not modify their physicochemical properties or intratubular disinfection of dentin contaminated with E. faecalis.
MATERIAL AND METHODS
The medications associated with cetrimide were divided into four Experimental Groups, according to Table 1. The sample size was determined based on a pilot study using G*Power software (Heinrich-Heine-Universitat), aiming for a power of 0.8 and an alpha error of 0.05. All tests were conducted by a sole proficient operator who was blinded to the study conditions.
pH
The intracanal medications were inserted into polyethylene tubes (n=10/group) measuring 10 mm long and 1.6 mm in internal diameter. Subsequently, the tubes were immersed in plastic receptacles containing 10 mL distilled water and kept in an oven at 37ºC, for time intervals of 1, 3, 7, 14, and 21 days. After each time interval of analysis, they were removed and placed in new receptacles with 10 mL distilled water for measuring the pH of the solution with a digital pH-meter (FiveEasy Plus FP20, Mettler Toledo®, Columbus, Ohio, USA), previously calibrated, at an ambient temperature of 25ºC. For the Control Group, pH values were read in distilled water without any sample immersed in it.
SOLUBILITY
Polyethylene tubes (n=7/group), measuring 10 mm in length and 2 mm in diameter, were filled according to the experimental groups and placed in an oven at 37ºC with 95% humidity for 24 hours. Subsequently, the samples were transferred to a desiccator for another 24 hours. The samples were then weighed using an analytical electronic balance (OHAUS Adventurer ®, New Jersey, USA) until the initial mass stabilized. To determine the final mass, the tubes were placed in plastic receptacles containing 7.5 mL of distilled water at 37ºC. After 14 days, the tubes were returned to the desiccator, and weighing was performed until the final mass stabilized. The solubility was calculated by finding the difference between the initial mass and final mass of each sample.
INTRATUBULAR DECONTAMINATION
FABRICATION, CONTAMINATION AND FILLING OF DENTIN TUBES
This study was approved by the University Ethics Committee (no. 35/2020). Bovine dentin tubes were fabricated following the methodology of Haapasalo et al. (1987) (2) with modifications. Uniradicular bovine teeth were sectioned at 1 mm and 4 mm from the cementoenamel junction to obtain dentin tubes measuring 3 mm in height. The tubes were prepared with a Gates-Glidden burr number 4 (Dentsply Maillefer, Ballaigues, Switzerland) to standardize the internal diameter at 1.1 mm and wall thickness at approximately 2 mm. Subsequently, the specimens were submitted to an ultrasonic bath with EDTA (Biodinâmica, Ibiporã, PR, Brazil), sterilized in an autoclave at 121°C for 20 minutes, and then randomly divided according to the Experimental Group and Control Group (Polyethylene glycol 400).
The procedures were conducted in a laminar flow chamber (VecoFlow Ltda, Campinas, SP, Brazil). Before contamination, the samples were immersed in a culture medium of Tryptic Soy broth (TSB) (Difco, Detroit, MI, USA) and subjected to an ultrasonic bath for 15 minutes to rehydrate the samples, allowing the bacterial suspension to penetrate the dentinal tubules. Subsequently, the specimens were placed in polyethylene tubes of the Eppendorf type, and 500 µL of bacterial inoculum of E. faecalis ATCC 29212 was added. The inoculum concentration was adjusted in a spectrophotometer to an optical density equivalent to 1x108 CFU mL-1. The tubes were centrifuged at speeds of 1400g, 2000g, 3600g, and 5000g (Centrifuge 5430; Eppendorf, Hamburg, Germany) for 5 minutes, repeated twice for each centrifugation process (25). Following the centrifugation procedures, the samples were incubated at 37°C. These steps were repeated after 48 hours for 5 days, with the culture medium TSB being renewed daily.
After the incubation period, the tubes were removed from the Eppendorf tubes, their external surfaces were decontaminated with 2.5% sodium hypochlorite and neutralized with 1% sodium thiosulfate. The canal of the tubes was irrigated with physiological solution, filled with 17% ethylenediamine tetraacetic acid (EDTA) for 3 minutes, and then irrigated with physiological solution. Subsequently, the tubes were dried with sterile gauze and filled with the medications. The Control Group was filled with Polyethylene Glycol 400. The samples were incubated in an oven at 37°C with 95% humidity for 3 days.
EVALUATION BY CONFOCAL LASER SCANNING MICROSCOPY
After 3 days, irrigation was performed using Phosphate buffered saline (PBS) to remove the medication with a #40 file (Dentsply Maillefer, Ballaigues, Switzerland). The tubes were cleaved longitudinally into two halves using an IsoMet 100 cutting machine (Buehler Ltda, Lake Bluff, IL, USA) under cooling, resulting in n=6 for each group. Subsequently, the were placed in a 24-well culture plate with 17% EDTA for 3 minutes and washed with sterile saline solution to remove the smear layer from sectioning. The samples were then treated with 30µL of Live/Dead BacLight Bacterial Viability solution (Molecular Probes, Inc, Eugene, OR, USA) for 15 minutes at room temperature, protected from light, and washed with PBS to remove excess dye. The samples were examined using a confocal laser scanning microscope (Leica TCS-SPE; Leica Microsystems GmbH, Mannheim, Germany) at 5X magnification, and the images were analyzed using Zeiss ZEN 2 lite software (Carl Zeiss, Germany). An area of approximately 220 cm² was standardized for all images. The program automatically determined the presence of viable and non-viable cells in the standardized area by calculating the intensity of the mean values of the green and red areas. The ratio of red to green fluorescence indicated the percentage of dead cells in each sample.
STATISTICAL ANALYSIS
The data on pH, solubility, and intratubular decontamination were analyzed using GraphPad Prism 6.01 software. Normality tests were conducted, followed by two-way analysis of variance (ANOVA) and Tukey tests with a significance level of 5%.
RESULTS
Medications resulted in higher pH values compared to the Control group (p<0.05). CAL and CAL/CTR exhibited higher pH values across all time intervals (p<0.05). CAL/CTR had the highest alkalinity after 1 day (p<0.05). CAL and CAL/CTR were similar after 3 and 7 days (p>0.05) and had higher pH values compared to BCT and BCT/CTR (p<0.05). CAL showed the greatest alkalinization potential at 14 and 21 days (p<0.05). BCT was similar to BCT/CTR after 14 days (p>0.05) (Table 2).
The solubility of CAL was comparable to CAL/CTR (p>0.05), while BCT and BCT/CTR had similar solubility values (p>0.05). CAL and CAL/CTR exhibited higher solubility values (p<0.05) (Table 2).
All medications resulted in higher percentages of non-viable cells compared to the Control group (Polyethylene glycol 400) (p<0.05). CAL/CTR, BCT, and BCT/CTR had lower percentages of non-viable cells than CAL (p<0.05) (Table 3) (Figure 1).
Table 1. Experimental Groups and their respective compositions and proportions.
|
Groups |
Composition |
Proportion – 99%:1% |
|
CAL¹ |
Calcium Hydroxide, Zinc Oxide, Colophony and Polyethylene Glycol 400* |
Ready for use |
|
CAL/CTR² |
* +1% CTR |
990mg/10mg |
|
BCT3 |
Calcium Silicate, Calcium Aluminate, Calcium Oxide, Resin Base, Calcium Tungstate, and Titanium Oxide** |
Ready for use |
|
BCT/CTR |
**+ 1% CTR |
990mg/10mg |
¹CAL (Calen®) *: S. S. White Artigos Dentários Ltda, Rio de Janeiro, RJ, Brazil; ²CTR (Cetrimide): Sigma-Aldrich Brasil Ltda., São Paulo, SP, Brazil; 3BCT (Bio-C® Temp) **: Angelus Industria de Produtos Odontológicos S/A, Londrina, PR, Brazil.
Table 2. Mean and standard deviation of the pH and solubility (% mass loss) observed in the different groups.
|
CAL |
CAL/CTR |
BCT |
BCT/CTR |
Control |
|
|
pH 1 day |
11.55±0.15ᵇ |
12±0.05ᵃ |
10.53±0.47ᶜ |
10.61±0.28ᶜ |
7.586±0.27 ͩ |
|
pH 3 days |
11.44±0.12ᵃ |
11.67±0.08ᵃ |
10.28±0.30ᶜ |
10.67±0.26ᵇ |
7.764±0.13 ͩ |
|
pH 7 days |
11.52±0.20ᵃ |
11.58±0.07ᵃ |
10.45±0.25ᵇ |
9.95±0.4ᶜ |
7.648±0.20 ͩ |
|
pH 14 days |
11.6±0.12ᵃ |
10.97±0.52ᵇ |
10.31±0.30ᶜ |
10.19±0.33ᶜ |
7.405±0.15 ͩ |
|
pH 21 days |
11.56±0.09ᵃ |
10.89±0.42ᵇ |
10.13±0.45ᶜ |
9.15±0.86 ͩ |
7.371± 0.23 ͤ |
|
Solubility |
20.01±1.17ᵃ |
18.27±2.78ᵃ |
14.15±3.12ᵇ |
12.73±1.25ᵇ |
----- |
Different letters in the same line represent statistically significant differences among groups in the same time interval.
CAL (Calen®); CTR (Cetrimide); BCT (Bio-C® Temp).
Table 3. Mean and standard deviation of the percentage (%) values of non-viable cells of Enterococcus faecalis after filing the tubes with the intracanal medications and Control.
|
CAL |
CAL/CTR |
BCT |
BCT/CTR |
Control |
|
40.99±3.26ᵃ |
32.02±4.76ᵇ |
30.67±2.12ᵇ |
34.15±2.78ᵇ |
13.62±2.32 ᶜ |
Different letters in the same line represent statistically significant differences among groups.
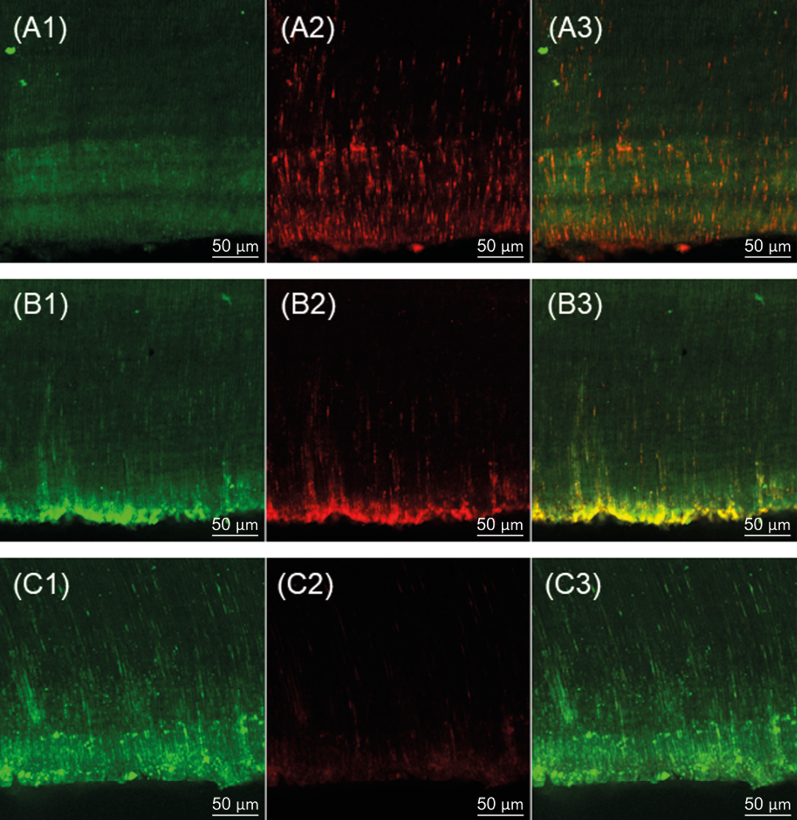
Figure 1. Confocal laser scanning microscopy images after contact of medications and polyethylene glycol 400 (control group) in dentin tubes contaminated with E. faecalis: (A) CAL; (B) BCT/CTR; (C) control group; (1) viable microorganisms; (2) non-viable microbial cells and (3) overlapping viable and non-viable cells. CAL (Calen®); CTR (Cetrimide); BCT (Bio-C® Temp).
DISCUSSION
The association of CTR is suggested to enhance the antibacterial properties of endodontic materials. Previous studies have evaluated CTR concentrations ranging from 0.2% (26) to 2.5% (27). In this study, a 1% concentration was utilized, similar to a study by Carbajal Mejia & Aguilar Arrieta (2016) (28), which showed effectiveness against E. faecalis using 1% CTR as an intracanal medication. The null hypothesis was partially accepted, as the association of 1% cetrimide (CTR) with the bioceramic medication, Bio-C® Temp (BCT), and the calcium hydroxide medication Calen® (CAL) did not affect the solubility and intratubular decontamination of BCT. However, it did change the pH at certain time intervals and reduced the efficacy of CAL against E. faecalis.
The association of CTR resulted in increased alkalinization for CAL on the first day and BCT on the third day, but lower alkalinization values were observed over 21 days. The vehicle present in medications is crucial for their physicochemical properties and clinical application (29). CTR may reduce the viscosity of irrigating solutions and the viscosity and pH of CAL over longer periods (30). Viscous vehicles such as Polyethylene glycol 400 in CAL promote prolonged ionic release, enhancing its biological and antimicrobial properties (10, 29). Therefore, CTR associated with CAL could have provided a higher level of initial ionic dissociation and afterward decrease its alkaline potential.
In this study, the solubility assessment followed the American National Standards Institute/American Dental Association (ANSI/ADA) guidelines for endodontic sealers, as there are currently no standardized specifications for intracanal medications. The solubility of materials is determined by the loss of mass after their immersion in water (31). The association of CTR with CAL and BCT did not change the solubility of the medications. Additionally, the solubility of reparative tricalcium silicate cement with zirconia oxide was not altered by the association with 0.2% and 0.4% of CTR (31). However, this material exhibited a setting reaction, which was not observed with BCT.
CAL exhibited higher solubility and pH values compared to BCT, supported by a study by Lopes et al. (2024) (6) that also demonstrated a superior alkalinizing potential for CAL relative to BCT. The pH of BCT showed a significant decrease after 1, 24, and 72 hours when compared to Ultracal® XS (UltraDent Product, Inc., Indaiatuba-SP, Brazil), a Ca(OH)₂-based medication (4). Similar results were reported by Capitanio et al. (2023) (6), who observed a lower alkalinizing capacity of BCT after 24 hours compared to Ultracal® XS. BCT contains a resin vehicle that inhibits setting during hydration reaction (9), resulting in lower solubility, reduced release of OH- ions, and consequently, lower alkalinization. Additionally, calcium silicate-based materials in the presence of humidity form Ca(OH)₂, leading to subsequent ionic dissociation and thereby increasing the pH of the medium (4).
In the present study, the addition of 1% CTR did not enhance intratubular decontamination of BCT and reduced the efficacy of CAL. This finding is in accordance with Alamri et al. (2023) (32), who reported that 0.5% CTR with calcium hydroxide paste compromised antibacterial activity against E. faecalis biofilm after 1 day. Incorporating CTR into endodontic materials may impede flow (33) and contact with dentin walls (34). Penã-Bengoa et al. (2023) (35) observed that Ultracal XS, medication based on Ca(OH)₂, had less depth and tubular penetration compared to BCT. Since Ca(OH)₂-based medications rely on direct contact with dentin walls and ionic dissociation for efficacy (36), the addition of CTR to CAL may have hindered its antibacterial action.
Another possible reason for the lack of improvement in the antimicrobial activity of the medications could be attributed to the fact that more significant results for the association with CTR were observed after longer exposure times, such as one and two weeks (32, 37). This effect may be due to the residual antimicrobial properties of CTR that become more pronounced over prolonged periods (21, 37). As a result, it is recommended that future studies should consider increasing the exposure time of the materials to better assess the full extent of CTR's efficacy.
The medication based on Ca(OH)₂, CAL, demonstrated higher effectiveness against E. faecalis in comparison to BCT. Studies have demonstrated that CAL and Ultracal® XS, both based on Ca(OH)₂, exhibited higher antibacterial and antibiofilm activity against E. faecalis than BCT (7, 12, 14), thus corroborating the results of our investigation. The lower effectiveness of BCT may be attributed to its reduced ionic release and consequent lower alkalinization potential, as observed in this study. Medications based on Ca(OH)₂ with either an aqueous or viscous vehicle showed better action against E. faecalis in intratubular decontamination, as analyzed by confocal laser scanning microscopy (38).
In this study, intratubular decontamination was assessed using confocal laser scanning microscopy with bovine dentin blocks, following the method proposed by Haapasalo & Orstavik (1986) (2). This technique enables three-dimensional analysis of images, distinguishing between living and dead cells and allowing visualization of microorganisms at different depths within dentinal tubules (39, 40). Microorganisms can form biofilms in these areas, making them more resistant to antimicrobial agents (11). However, this method has limitations, such as challenges in standardizing contamination levels across specimens and potential inaccuracies in sampling and cultivation (32). Additional antimicrobial analysis methods, like colony-forming unit counting, should be considered to assess the efficacy of different antimicrobial agents. Using multispecies biofilms can also help simulate more realistic in vivo conditions.
Considering the results obtained in the present study, the proportion of 1% CTR that was utilized in this study did not result in an enhancement of the intratubular decontamination efficacy of the medications against E. faecalis. Consequently, it is important that further research be conducted to investigate the effects of varying proportions and to examine the impact of extending the exposure times of the pastes when interacting with the biofilm. Such research could potentially yield more effective decontamination strategies.
CONCLUSION
The association of 1% cetrimide did not increase the intratubular decontamination of the bioceramic medications Bio-C® Temp and decreased the antibacterial effectiveness of calcium hydroxide Calen®. The calcium hydroxide-based medication in a viscous vehicle exhibited higher alkaline potential, solubility, and intratubular decontamination.
CONFLICT OF INTEREST: The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTION STATEMENT: Conceptualization and design: L.B.P., C.S.L., J.A.P., M.T.-F., and J.M.G.-M.; Literature review: L.B.P., C.S.L. and J.A.P.; Methodology and validation: L.B.P., C.S.L., J.A.P., M.T.-F., and J.M.G.-M.; Formal analysis: L.B.P., C.S.L., J.A.P. ; Investigation and Data Collection: L.B.P., C.S.L., J.A.P., and M.T.-F.; Resources: L.B.P., C.S.L., M.T.-F., and J.M.G.-T.; Data Analysis and Interpretation: L.B.P., C.S.L., J.A.P., M.T.-F., and J.M.G.-M.; Writing-Original Draft Preparation: L.B.P., C.S.L. and J.A.P.; Writing-Review & Editing: M.T.-F., and J.M.G.-T.; Supervision: M.T.-F., and J.M.G.-T.; Project administration and funding acquisition: M.T.-F., and J.M.G.-T.
REFERENCES
1. Siqueira J.F. Jr, Rôças I.N. Clinical implications and microbiology of bacterial persistence after treatment procedures. J Endod. 2008 Nov; 34 (11): 1291-1301.
2. Haapasalo M., Orstavik D. In vitro infection and disinfection of dentinal tubules. J Dent Res. 1987 Aug; 66 (8): 1375-9.
3. Villa N., Santos V.V.D., Costa U.M.D., Mendes A.T., Duarte P.H.M., Rosa R.A.D., Pereira J.R., Só M.V.R. A New Calcium Silicate-Based Root Canal Dressing: Physical and Chemical Properties, Cytotoxicity and Dentinal Tubule Penetration. Braz Dent J. 2020 Nov-Dec; 31 (6): 598-604.
4. Oliveira L.V., de Souza G.L., da Silva G.R., Magalhães T.E.A., Freitas G.A.N., Turrioni A.P., de Rezende Barbosa G.L., Moura C.C.G. Biological parameters, discolouration and radiopacity of calcium silicate-based materials in a simulated model of partial pulpotomy. Int Endod J. 2021 Nov; 54 (11): 2133-2144.
5. Capitanio B.L., Hashizume L.N., Kuga M.C., Oliveira E.C.G., Rosa R.A.D., Só G.B., Só M.V.R. Analysis of pH, calcium ion release, and energy dispersive spectroscopy of a bioceramic root canal dressing. Braz Dent J. 2023 Jul-Aug; 34 (4): 54-61.
6. Lopes C.S., Ferrari Esteves Torres F., Faria G., Sasso-Cerri E., Guerreiro-Tanomaru J.M., Tanomaru-Filho M., Cerri P.S. Calcium silicate-based intracanal medication: Physicochemical properties and effectiveness of techniques for removing medication from the human root canal. Eur Endod J. 2024 Dec 20; 9 (4): 374-382.
7. Oliveira L.V., da Silva G.R., Souza G.L., Magalhães T.E.A., Barbosa G.L.R., Turrioni A.P., Moura C.C.G. A laboratory evaluation of cell viability, radiopacity and tooth discoloration induced by regenerative endodontic materials. Int Endod J. 2020 Aug; 53 (8): 1140-1152.
8. Lopes C.S., Delfino M.M., Tanomaru-Filho M., Sasso-Cerri E., Guerreiro-Tanomaru J.M., Cerri P.S. Hepatic enzymes and immunoinflammatory response to Bio-C Temp bioceramic intracanal medication implanted into the subcutaneous tissue of rats. Sci Rep. 2022 Feb 18; 12 (1): 2788.
9. Lopes C.S., Delfino M.M., Tanomaru-Filho M., Sasso-Cerri E., Guerreiro-Tanomaru J.M., Cerri P.S. Bioactive potential of Bio-C Temp demonstrated by systemic mineralization markers and immunoexpression of bone proteins in the rat connective tissue. J Mater Sci Mater Med. 2024 Feb 14; 35 (1): 13.
10. Escobar P.M., Silva-Sousa A.C., Camargo R.V., Simões-Carvalho M., Silva-Sousa Y.T., Mazzi-Chaves J.F., DE-Deus G., Sousa-Neto M.D. Influence of bioceramic intracanal medication on the bond strength of bioceramic root canal sealer. Braz Oral Res. 2023 May 29; 37: e056.
11. de Campos I.V.B., Vieira W.A., de Almeida R.F., Gabriel P.H., Marciano M.A., Gomes B.P.F.A., de-Jesus-Soares A. In Vitro Dental Discoloration Provoked by Intracanal Calcium Silicate-based Dressing Used for Regenerative Endodontic Procedures: An One-year Spectrometric Analysis. J Endod. 2023 Jul; 49 (7): 846-851.
12. Patri G., Bansal S., Lath H., Chatterjee I., Majee N., Sinha Y. Comparative evaluation of the antibacterial efficacy of two experimental calcium silicate-based intracanal medicaments: An in-vitro study. J Conserv Dent Endod. 2024 Apr; 27 (4): 419-423.
13. Guerreiro J.C.M., Ochoa-Rodrígez V.M., Rodrigues E.M., Chavez-Andrade G.M., Tanomaru-Filho M., Guerreiro-Tanomaru J.M., Faria G. Antibacterial activity, cytocompatibility and effect of Bio-C Temp bioceramic intracanal medicament on osteoblast biology. Int Endod J. 2021 Jul; 54 (7): 1155-1165.
14. Siqueira P.C., Soares L.D.C.C., Wanderley K.R.D.M., Deus L.B., Melo H.V., Ávila M.P.A., Estrela L.R.A., Estrela C.R.A. The impact of intracanal medicaments on crown color, pH, and antimicrobial activity: a comparative study. Braz Oral Res. 2025 Mar 10; 39: e029.
15. Mohammadi Z., Dummer P.M. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int Endod J. 2011 Aug; 44 (8): 697-730.
16. Lima R.K., Guerreiro-Tanomaru J.M., Faria-Júnior N.B., Tanomaru-Filho M. Effectiveness of calcium hydroxide-based intracanal medicaments against Enterococcus faecalis. Int Endod J. 2012 Apr; 45 (4): 311-6.
17. Pimenta H.C., Violante I.M., Musis C.R., Borges Á.H., Aranha A.M. In vitro effectiveness of Brazilian brown propolis against Enterococcus faecalis. Braz Oral Res. 2015; 29: S1806-83242015000100255.
18. McDonnell G., Russell A.D. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999 Jan;12 (1): 147-79. doi: 10.1128/CMR.12.1.147. Erratum in: Clin Microbiol Rev 2001 Jan; 4 (1): 227.
19. Ruiz-Linares M., Ferrer-Luque C.M., Arias-Moliz T., de Castro P., Aguado B., Baca P. Antimicrobial activity of alexidine, chlorhexidine and cetrimide against Streptococcus mutans biofilm. Ann Clin Microbiol Antimicrob. 2014 Aug 20;13: 41.
20. Arias-Moliz M.T., Ferrer-Luque C.M., González-Rodríguez M.P., Valderrama M.J., Baca P. Eradication of Enterococcus faecalis biofilms by cetrimide and chlorhexidine. J Endod. 2010 Jan; 36 (1): 87-90.
21. Baca P., Junco P., Arias-Moliz M.T., González-Rodríguez M.P., Ferrer-Luque C.M. Residual and antimicrobial activity of final irrigation protocols on Enterococcus faecalis biofilm in dentin. J Endod. 2011 Mar; 37 (3): 363-6.
22. Wang Z., Shen Y., Ma J., Haapasalo M. The effect of detergents on the antibacterial activity of disinfecting solutions in dentin. J Endod. 2012 Jul; 38 (7): 948-53.
23. Giardino L., Ambu E., Becce C., Rimondini L., Morra M. Surface tension comparison of four common root canal irrigants and two new irrigants containing antibiotic. J Endod. 2006 Nov; 32 (11): 1091-3.
24. Bailón-Sánchez M.E., Baca P., Ruiz-Linares M., Ferrer-Luque C.M. Antibacterial and anti-biofilm activity of AH plus with chlorhexidine and cetrimide. J Endod. 2014 Jul; 40 (7): 977-81.
25. Andrade F.B., Arias M.P., Maliza A.G., Duarte M.A., Graeff M.S., Amoroso-Silva P.A., Midena R.Z., Moraes I.G. A new improved protocol for in vitro intratubular dentinal bacterial contamination for antimicrobial endodontic tests: standardization and validation by confocal laser scanning microscopy. J Appl Oral Sci. 2015 Nov-Dec; 23 (6): 591-8.
26. Valverde M.E., Baca P., Ceballos L., Fuentes M.V., Ruiz-Linares M., Ferrer-Luque C.M. Antibacterial efficacy of several intracanal medicaments for endodontic therapy. Dent Mater J. 2017 May 31; 36 (3): 319-324.
27. Deveci C., Tuzuner T., Cinar C., Odabas M.E., Buruk C.K. Short-term antibacterial activity and compressive strength of biodentine containing chlorhexidine/cetirimide mixtures. Niger J Clin Pract. 2019 Feb; 22 (2): 227-231.
28. Carbajal Mejía J.B., Aguilar Arrieta A. Reduction of viable Enterococcus faecalis in human radicular dentin treated with 1% cetrimide and conventional intracanal medicaments. Dent Traumatol. 2016 Aug; 32 (4): 321-7.
29. Fava L.R., Saunders W.P. Calcium hydroxide pastes: classification and clinical indications. Int Endod J. 1999 Aug; 32 (4): 257-82.
30. Mohan R.P., Pai A.R. The comparison between two irrigation regimens on the dentine wettability for an epoxy resin based sealer by measuring its contact angle formed to the irrigated dentine. J Conserv Dent. 2015 Jul-Aug; 18 (4): 275-8.
31. Rodrigues G.B., Tanomaru-Filho M., Chavez-Andrade G.M., Esteves F.F., Guerreiro-Tanomaru J.M. Physicochemical properties and antibiofilm activity of tricalcium silicate cement and its association with cetrimide. Odovtos-Int J Dental SC. 2022 Jan-Apr; 24: 113-121.
32. Alamri H.M., Liu H., Zhang D., Shen Y., Haapasalo M. An In Vitro Study: Does Adding Iodine Potassium Iodide and Cetrimide to Calcium Hydroxide Paste Enhance Its Antimicrobial Effect Against Oral Biofilms? Cureus. 2023 Dec 27; 15 (12): e51203.
33. Ruiz-Linares M., Bailón-Sánchez M.E., Baca P., Valderrama M., Ferrer-Luque C.M. Physical properties of AH Plus with chlorhexidine and cetrimide. J Endod. 2013 Dec; 39 (12): 1611-4.
34. D'Costa V.F., Rodrigues A.L., Bangera M.K., Bhat P.A., Rai R.U. A Confocal Microscopic Study on Percentage Penetration of Different Sealers into Dentin. J Pharm Bioallied Sci. 2021 Jun;13 (Suppl 1): S725-S730.
35. Peña-Bengoa F., Magasich M.C., Bustamante D., Wastavino C., Niklander S.E., Cáceres C. Effect of Ultrasonic Activation on Dentinal Tubule Penetration of Bio-C Temp and Ultracal XS: A Comparative CLSM Assessment. Eur Endod J. 2023 Aug; 8 (4): 268-273.
36. Turk B.T., Sen B.H., Ozturk T. In vitro antimicrobial activity of calcium hydroxide mixed with different vehicles against Enterococcus faecalis and Candida albicans. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009 Aug; 108 (2): 297-301.
37. Ferrer-Luque C.M., Conde-Ortiz A., Arias-Moliz M.T., Valderrama M.J., Baca P. Residual activity of chelating agents and their combinations with cetrimide on root canals infected with Enterococcus faecalis. J Endod. 2012 Jun; 38 (6): 826-8.
38. Pereira T.C., da Silva Munhoz Vasconcelos L.R., Graeff M.S.Z., Ribeiro M.C.M., Duarte M.A.H., de Andrade F.B. Intratubular decontamination ability and physicochemical properties of calcium hydroxide pastes. Clin Oral Investig. 2019 Mar; 23 (3): 1253-1262.
39. Pereira T.C., Vasconcelos L.R., Graeff M.S., Duarte M.A., Bramante C.M., Andrade F.B. Intratubular disinfection with tri-antibiotic and calcium hydroxide pastes. Acta Odontol Scand. 2017 Mar; 75( 2): 87-93.
40. Arias M.P., Maliza A.G., Midena R.Z., Graeff M.S., Duarte M.A., Andrade F.B. Effect of ultrasonic streaming on intra-dentinal disinfection and penetration of calcium hydroxide paste in endodontic treatment. J Appl Oral Sci. 2016 Nov-Dec; 24 (6): 575-581.
 Odovtos -Int J Dent Sc endoses to CC-BY-NC-SA 4.0.
Odovtos -Int J Dent Sc endoses to CC-BY-NC-SA 4.0.