Odovtos-International Journal of Dental Sciences (Odovtos-Int. J. Dent. Sc.), Online First, 2025. ISSN: 2215-3411
https://doi.org/10.15517/v30z1132

https://revistas.ucr.ac.cr/index.php/Odontos
BASIC RESEARCH:
Evaluation of Colonization and Biofilm Formation of E. faecalis over G-MTA, W-MTA and Biodentine. In vitro study
Evaluación de la colonización y la formación de biofilm de E. faecalis sobre G-MTA, W-MTA y Biodentine. Estudio in vitro
Alejandra Ruiz-Flores¹ https://orcid.org/0009-0004-1442-5031
Ana Maria González-Amaro² https://orcid.org/0000-0002-6375-9642
Norma Verónica Zavala-Alonso¹ https://orcid.org/0000-0001-8822-185X
Francisco Javier Gutiérrez-Cantú3 https://orcid.org/0000-0001-7220-5791
Jairo Mariel-Cárdenas3 https://orcid.org/0000-0002-4733-7271
Ricardo Oliva-Rodríguez² https://orcid.org/0000-0002-1797-1773
Abraham I. Muñoz-Ruiz¹ https://orcid.org/0000-0003-2522-3617
¹Postgraduate Dental Science Program, Faculty of Dentistry, UASLP, San Luis Potosí, SLP, México.
²Endodontics Postgraduate Program, Faculty of Dentistry, UASLP, San Luis Potosí, SLP, México.
3Department of Morphology, Faculty of Dentistry, UASLP, San Luis Potosí, SLP, México.
Correspondence to: Abraham I. Muñoz-Ruiz - abraham.munoz@uaslp.mx
Received: 22-I-2025 Accepted: 4-IX-2025
ABSTRACT: Apicectomy is a procedure indicated for the treatment of non-healing apical periodontitis after non-surgical Root Canal Treatment or certain clinical conditions; the procedure consists in the elimination of the last 3 mm of the RCS and creation of an apical preparation to finally seal it with a root-end filling material, like bioceramics. It has been reported that bioceramic materials (Gray-MTA, White-MTA and Biodentine) have adequate antibacterial properties. The aim of the study is to evaluate the colonization capacity and biofilm formation of E. faecalis over the surface of bioceramic materials In vitro. 180 dentin disks were employed and distributed in 3 experimental groups (G-MTA+E. faecalis, W-MTA E. faecalis and Biodentine+E. faecalis) and 3 control groups (G-MTA, W-MTA and Biodentine), the surface area covered by biofilm and microorganisms were evaluated by SEM. All samples of experimental groups showed a rough and irregular surface due to microorganism colonization and biofilm formation. G-MTA group showed better antimicrobial properties than W-MTA and Biodentine. The complete elimination and removal of damaged and contaminated periapical tissues during the surgical treatment is critical for long-term success.
KEYWORDS: Apicectomy, Root-End Filling Material, Biodentine, Gray-MTA, White-MTA.
RESUMEN: La apicectomía es un procedimiento indicado para el tratamiento de lesiones y periodontitis apical persistente después de un tratamiento de conductos; el procedimiento consiste en la eliminación de los últimos 3 mm apicales y posterior creación de una preparación para finalmente sellarlo con un material de obturación. Se ha reportado que los materiales biocerámicos (MTA-Gris, MTA-Blanco y Biodentine) tienen propiedades antibacterianas adecuadas. El objetivo de este estudio es evaluar la capacidad de colonización y formación de biofilm de E. faecalis sobre la superficie de materiales biocerámicos in vitro. Se emplearon 180 discos de dentina aleatorizados en 3 grupos experimentales (G-MTA+E. faecalis, W-MTA E. faecalis y Biodentine+E. faecalis) y 3 grupos control (G-MTA, W-MTA y Biodentine), El área/superficie cubierta por biofilm y microorganismos fue evaluada mediante SEM. Todas las muestras de los grupos experimentales mostraron una superficie rugosa e irregular debido a la colonización de microorganismos y la formación de biopelículas. El grupo de MTA-Gris mostró mejores propiedades antimicrobianas que MTA-Blanco y Biodentine. La eliminación y extirpación completa de los tejidos periapicales dañados y contaminados durante el tratamiento quirúrgico es fundamental para el éxito a largo plazo.
PALABRAS CLAVE: Apicectomía, Material de retro-obturación, Biodentine, MTA-Gris, MTA-Blanco.
INTRODUCTION
The preliminary purpose of all endodontic procedure is to eliminate necrotic tissues and infective bacteria (1). The success of non-surgical root canal treatment (RCT) ranges up to 98%, even when it is a predictable treatment, certain unfavorable clinical conditions and the root canal system (RCS) complexity (2) lead to the preservation of certain pathologic process (3).
Apicectomy is a procedure indicated for the treatment of non-healing apical periodontitis after non-surgical RCT or certain clinical conditions (4), being the most common indications: intra-or extra-radicular infections; persistent periapical pathology that fails to resolve after non-surgical RCT (1,3); periapical pathology with prosthodontic or conservative restoration proven to be difficult to remove; a radiolucent lesion measuring over 8 to 10 mm in diameter; symptomatic gutta-percha overfilling, or presence of a foreign body not amenable to orthograde removal (3);
The main objective of apicectomy is to seal the RCS, thereby enabling healing by forming a barrier between the irritants within the confines of the affected root and the periapical tissues (3) to prevent the invasion of bacteria and their by-products (5). The procedure consists in the elimination of the last 3 mm of the RCS (2,3) to remove most of the apical ramifications and lateral canals (2) and to avoid reinfection and therefore the recurrence of the lesion (3), also, part of the intracanal endodontic-filling material must be removed to create a cavity with parallel walls and coincident with the anatomic outline of the RCS (2), to finally seal the cavity with an adequate root-end filling material (5). Nowadays the success rate of surgical endodontic therapy ranges up to 93% at 1 to 10 years, and depends on different factors, including: case selection, host response, diminished bacteria, removal of necrotic and infected tissue, local anatomy, root end-filling material applied, sealing and operator technique (6).
There are several root-end filling materials used during apicectomy (2). Mineral Trioxide Aggregate (MTA) is an ideal root-end filling material since it promotes the formation of a physical and biological seal (3), another recommended material is Biodentine since it shows adequate sealing (7) and holds antibacterial properties due to alkalization of the environment, which leads to the disinfection of adjacent hard and soft tissue structures (5); when these materials interact with new-generation endodontic sealers like, AH Plus Bioceramic Sealer, EndoSequence BC Sealer HiFlow, C-Root SP, and GuttaFlow Bioseal, guarantee an adequate sealing of the RCS (8).
It has been previously reported that Gray-MTA (G-MTA), White-MTA (W-MTA) and Biodentine have adequate antibacterial properties, but until now there is not enough literature related to the biofilm formation post-surgical RCT over the surface of these materials.
The aim of the study is to evaluate the interaction, colonization, and formation of biofilm (Enterococcus faecalis) over the surface of bioceramic materials used as root end-filling sealers In vitro.
Materials and Methods
This study was authorized by the Research Ethics Committee of the Faculty of Stomatology, UASLP, Mexico (CEI-FE-035-020).
Thirty-seven single-root human premolars extracted due to orthodontic reasons were used in the present study, the samples were placed in sterile saline solution and stored at -4°C until use. The crowns were removed to obtain a root length of 15 mm; then the roots were cut to obtain 3-4 mm dentin disks (180 samples). In each sample a standard preparation was performed using an high speed carbide bur #4 (SS White, New Jersey, USA); then the samples were disinfected according to Hapassalo’s protocol (9) (NaClO 5.25%+ultrasound-Biosonic UC50 [30 htz, 4 minutes]; EDTA 17 %+ultrasound-Biosonic UC50 [30 htz, 4 minutes]; distilled water+ultrasound-Biosonic UC50 [30 htz, 4 minutes]; and a sterilization process (autoclave equipment [121˚C, 15 minutes]).
The 180 samples were randomized and distributed in each group as follow: Group 1 [G-MTA (Angelus, Londrina, Paraná Brasil)+E. faecalis biofilm] (30 dentine disks); Group 2 [W-MTA (Angelus, Londrina, Paraná Brasil)+E. faecalis biofilm] (30 dentine disks); Group 3 [Biodentine (Septodont, Saint-Maurdes-Fosse´s Cedex, Francia)+E. faecalis biofilm] (30 dentine disks); Group 4 [G-MTA (Angelus, Londrina, Paraná Brasil)] (30 dentine disks); Group 5 [W-MTA(Angelus, Londrina, Paraná Brasil)] (30 dentine disks); Group 6 [Biodentine (Septodont, Saint-Maurdes-Fosse´s Cedex, Francia)] (30 dentine disks).
An E. faecalis strain isolated from a patient with apical periodontitis was used for this study; to corroborate the purity of the microorganism an API-20 strep test was performed. An E. faecalis solution was prepared (0.5 McFarland scale). Samples of Group 1, Group 2, and Group 3 were incubated with 200 µL of E. faecalis solution [0.5 McFarland] + 29.8 ml of BHI medium (37 °C, 100% humidity); samples of Group 4, Group 5, and Group 6, were cultured using 30 mL of sterile BHI medium (37 °C, 100% humidity). The bacterial growth medium was replaced every 72 hours, 21 days.
The samples were prepared for Scanning Electron Microscopy (SEM), the SEM observation was performed at 2500x; A four-score scale system (10,11) based on the percentage area covered by biofilm and microorganisms was used; The scores were defined as follow: 1=Biofilm and microbial cells covering less than 5% of the surface dentine, 2=Biofilm and microbial cells covering 5%-33% of the surface dentine, 3=Biofilm and microbial cells covering 34%-66% of surface dentine, 4=Biofilm and microbial cells covering 67%-100% of surface dentine; the samples were classified by a calibrate blind evaluator. A statistical analysis was performed (IBM SPSS Statistics 21); a Shapiro-Wilk, Kruskal-Wallis and Mann-Whitney U test were done.
Results
In SEM images was observed that all samples in the experimental groups (Groups 1, 2, 3) showed a rough and irregular surface due to microorganism colonization and biofilm formation, in which could be observed coccoid forms compatibles with E. faecalis, the coccoid forms were found singles, in agglomerations like-chains, and amorphous accumulations of clusters surrounded by an extracellular polymeric matrix, which indicate a dynamic metabolic activity. In the present study all root-end filling materials employed showed microorganism adherence on their surface and biofilm formation could be identified in different stages (adherence of microorganisms to surfaces; micro-colonization or formation of colonies; biofilm maturation; growth, dispersion and shedding of microorganisms) (Figure 1).
According to Kruskal-Wallis test the groups behave differently from each other (P=0.0001) in which Biodentine and W-MTA showed the most bacteria contamination (Figure 2). The Mann-Whitney U tests determines that there is significant statistical difference between paired groups: Group 1-Group 2 [G-MTA-W-MTA] (P=0.003); Group 1-Group 3 [G-MTA - Biodentine] (P=0.0001); and there is not significant statistical difference between paired groups: Group 2-Group 3 [W-MTA-Biodentine] (P=0.163). Based on the current results the G-MTA Group showed better antimicrobial properties compared to W-MTA and Biodentine (Figure 2).
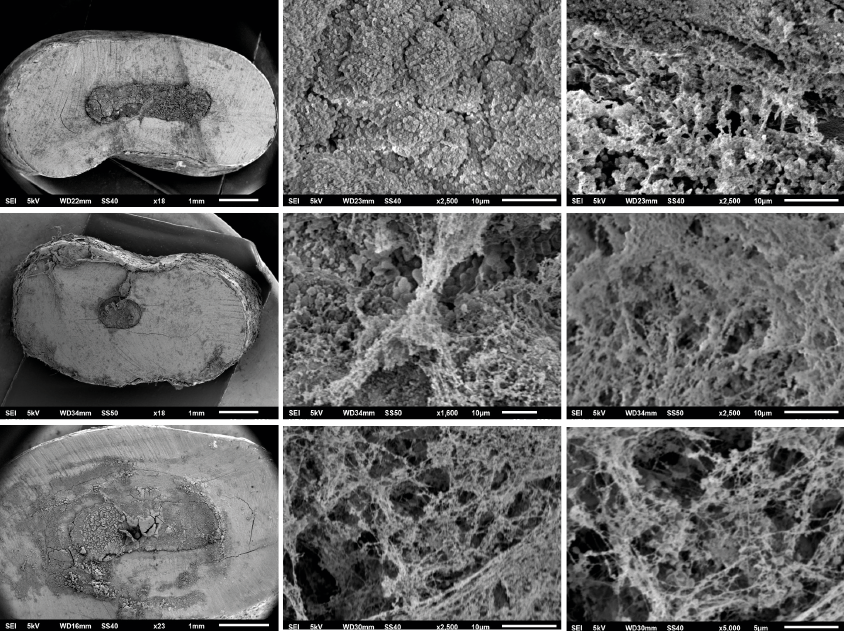
Figure 1. SEM Images. [A, B, C] G-MTA; [D,E,F] W-MTA; [G,H,I] Biodentine. A rough and irregular surface due to microorganism colonization and biofilm formation over the surface of G-MTA, W-MTA and biodentine can be observed, E. faecalis were found singles, in agglomerations like-chains, and amorphous accumulations of clusters surrounded by an extracellular polymeric matrix.

Figure 2. Plot Box. Experimental Groups behave differently compared to control groups. G-MTA showed the least amount of surface contaminated by bacteria compared with W-MTA and Biodentine.
Discussion
In the present study, the capacity of E. faecalis to adhere, colonize, and form biofilm over the surface of bioceramic materials (G-MTA, W-MTA and Biodentine) in vitro was evaluated; a modified simulated controlled protocol (5) was employed to carry out the experiments. According to the SEM images, E. faecalis has the capacity to adhere to the surface of the three evaluated bioceramic materials, forming an extracellular polymeric matrix (Biofilm), which confirms the metabolic activity of the remaining microorganism even after the disinfection protocols and apical seal; this suggests that reinfection of the periapical structures is possible even when antibacterial materials are employed to perform the root-end filling obturation during the apical surgery, therefore, the removal and disinfection of the periapical tissues is critical for the success of the treatment.
Apicectomy has always been considered as last option prior to tooth extraction (3), nowadays it is a technique that has evolved and shown good and predictable results for the healing of many periapical lesions (3) with a success rate up to 93% at 1 to 10 years (6); the procedure aims to reduce/eliminate infected tissues, persistent bacteria and its by-products from the RCS and periapical tissues (3); the success of periapical surgery is usually determined by imaging, clinical signs and symptoms (3), the prognosis depends on different factors (6), being one of the most relevant the root-end filling material applied; an ideal root-end filling material should provide an hermetic seal (12) to prevent the bacterial colonization and ensuing leakage of bacterial by-products into the peri-radicular tissues (5), also, should be non-toxic, non-carcinogenic, biocompatible and dimensionally stable (12). Nowadays, bioceramic materials (W-MTA, G-MTA, Biodentine) are considered the main option as filling material in apical surgery due to its characteristics such as biocompatibility, seal and the ability to prevent communication between periradicular tissues and RCS; moreover, it has been proven that bioceramic materials induce a positive biological response (13); also their antimicrobial and antifungal properties have been studied showing favorable results, properties usually attributed to the ability to release calcium ions, enhancing its ability to create alkaline environments (14-16).
Bacterial colonization is an active process carried out by a polymicrobial group in constant communication and interaction, the most frequent microorganisms in endodontic persistent infections are facultative anaerobic, being E. faecalis (due to its ability to survive, adhere to surfaces and form biofilm) one of the main microorganisms identified (24-77%) in isolated cultures of non-surgical endodontic treatment failures, (5,17,18), it is highly recalcitrant due to its ability to withstand alkaline conditions and glucose starvation, thus, it is prone to cause persistent infections, therefore, E. faecalis biofilms are considered an appropriate model for evaluating bacterial colonization (5) and have been used in multiple studies (5,19-23).
George et al. (24) evaluated biofilm formation of E. faecalis under two different environments (nourished medium and nutritional stress) at 21 days; according to data, biofilm development was carried out under both environments, biofilm formed with a nourished medium present a more complex and well-defined structure than biofilm under nutritional stress, which showed disperse and a non-compact structure. In the present study, at 21 days were identified multiple coccoid forms, found singles, in agglomerations, and amorphous accumulations of clusters surrounded by an extracellular polymeric matrix (biofilm) with communication channels, corroborating previous reported data. Bhavana et al. (14), evaluated the interaction of microorganisms (S. mutans, E. faecalis, E. coli, Candida albicans) with Biodentine, MTA and Glass Ionomer, and observed that Biodentine and MTA has a better antimicrobial properties against microorganisms than Glass ionomer, with no statical difference between Biodentine and MTA. The results obtained in the present study differ, since G-MTA showed less surface cover by biofilm than W-MTA and Biodentine. In another study, Kim et al. (19) carried out a study in which MTA did not show an adequate antimicrobial activity against E. faecalis, in the current study this data was confirmed since the SEM images showed that G-MTA and W-MTA groups had a full surface cover by biofilm, including the material-dentin interface. Khalid AL-Hezaimi et al. (25) evaluated the antimicrobial effect of MTA, and reported that E. faecalis, do not growth over G-MTA in the periods tested (24, 48 and 72 hours), but there was growth over W-MTA, corroborating that G-MTA has better antimicrobial properties than W-MTA.
Understanding the pathological process following endodontic surgery requires an experimental model which should enable not only to assess the ability of the root-end filling to seal and prevent bacterial migration (5) but also to track and quantify how the microbial colonization is carried out. According to the current protocol/model is possible to obtain an objective data related to the colonization of the root-end filling material surface. Based on current data, the complete elimination contaminated peri-apical tissues during the surgical endodontic treatment is critical for success.
Conclusions
Despite the antimicrobial properties attributed to bioceramic materials, the present study showed the capability of adhesion, proliferation, and biofilm formation of E. faecalis over the surface of evaluated bioceramic root-end filling materials. According to SEM images all materials showed an irregular, porous, rough surface, which contribute to adhesion and propagation of microorganisms; among the evaluated materials, G-MTA showed the least amount of surface contaminated by bacteria compared with W-MTA and Biodentine. Microorganisms have evolved and created adaptation mechanisms which help their survival and development; furthermore, the complete elimination and removal of damaged and contaminated peri-apical tissues during the surgical treatment is critical for success.
AUTHOR CONTRIBUTION STATEMENT: Conceptualization and design: A.I.M.R. and R.O.R.; Literature review: A.R.F. and A.I.M.R.; Methodology and validation: A.M.G.A. and A.I.M.R.; Formal analysis: F.J.G.C.; Investigation and data collection: J.M.C. and R.O.R.; Resources: A.M.G.A.; Data analysis and interpretation: A.I.M.R., J.M.C. and F.J.G.C.; Writing-original draft preparation: A.R.F. and R.O.R.; Writing-review & editing: F.J.G.C. and A.I.M.R.; Supervision: N.V.Z.A.; Project administration: A.I.M.R. and R.O.R.; Funding acquisition: A.M.G.A. and A.I.M.R.
REFERENCES
1. Alghamdi F., Alhaddad A.J., Abuzinadah S. Healing of Periapical Lesions After Surgical Endodontic Retreatment: A Systematic Review. Cureus. 2020; 12 (2): 1-9.
2. Kim S., Kratchman S. Modern Endodontic Surgery Concepts and Practice: A Review. J Endod. 2006; 32 (7): 601-23.
3. Serrano-Giménez M., Sánchez-Torres A., Gay-Escoda C. Prognostic factors on periapical surgery: A systematic review. Med Oral Patol Oral Cir Bucal. 2015; 20 (6): e715-22.
4. Kohli M.R., Berenji H., Setzer F.C., Lee S.M., Karabucak B. Outcome of Endodontic Surgery: A Meta-analysis of the Literature-Part 3: Comparison of Endodontic Microsurgical Techniques with 2 Different Root-end Filling Materials. J Endod. 2018; 44 (6): 923-31.
5. Tsesis I., Elbahary S., Venezia N.B., Rosen E. Bacterial colonization in the apical part of extracted human teeth following root-end resection and filling: a confocal laser scanning microscopy study. Clin Oral Investig. 2018; 22 (1): 267-74.
6. Chércoles-Ruiz A., Sánchez-Torres A., Gay-Escoda C. Endodontics, Endodontic Retreatment, and Apical Surgery Versus Tooth Extraction and Implant Placement: A Systematic Review. J Endod. 2017; 43 (5): 679-86.
7. Malkondu Ö., Kazandaǧ M.K., Kazazoǧlu E. A review on biodentine, a contemporary dentine replacement and repair material. Biomed Res Int. 2014; 1-10.
8. Zanza A., Reda R., Vannettelli E., Donfrancesco O., Relucenti M., Bhandi S., et al. The Influence of Thermomechanical Compaction on the Marginal Adaptation of 4 Different Hydraulic Sealers: A Comparative Ex Vivo Study. J Compos Sci. 2023; 7 (1).
9. Haapasalo M., Ørstavik D. In vitro Infection and Disinfection of Dentinal Tubules. J Dent Res. 1987; 66 (8): 1375-9.
10. Ordinola-Zapata R., Bramante C.M., Aprecio R.M., Handysides R., Jaramillo D.E. Biofilm removal by 6% sodium hypochlorite activated by different irrigation techniques. Int Endod J. 2014; 47 (7): 659-66.
11. Parolia A., Kumar H., Ramamurthy S., Madheswaran T., Davamani F., Pichika M.R., et al. Effect of propolis nanoparticles against enterococcus faecalis biofilm in the root canal. Molecules. 2021; 26 (3): 1-17.
12. Torabinejad M., Pitt Ford T.R. Root end filling materials: A review. Endod Dent Traumatol. 1996; 12 (4): 161-78.
13. Trujillo-hernández M., Flores-ventura R.E., Suárez-Porras A., García-González L., Hernández-Torres J., Zamora-Peredo L. Estudio comparativo de la bioactividad de dos materiales biocerámicos Comparative Study of the Bioactivity of Two Bioceramic Materials. ODOVTOS-International J Dent Sci. 2019; 2 (21): 73-81.
14. Bhavana V., Chaitanya K.P., Gandi P., Patil J., Dola B., Reddy R.B. Evaluation of antibacterial and antifungal activity of new calcium-based cement (Biodentine) compared to MTA and glass ionomer cement. J Conserv Dent. 2015; 18 (1): 44-6.
15. Hiremath G.S., Kulkarni R.D., Naik B.D. Evaluation of minimal inhibitory concentration of two new materials using tube dilution method: An in vitro study. J Conserv Dent. 2015;18 (2): 159-62.
16. Abusrewil S.M., McLean W, Scott J.A. The use of Bioceramics as root-end filling materials in periradicular surgery: A literature review. Saudi Dent J. 2018; 30 (4): 273-82.
17. Song M., Kim S.G., Lee S.J., Kim B., Kim E. Prognostic factors of clinical outcomes in endodontic microsurgery: A prospective study. J Endod. 2013; 39 (12): 1491-7.
18. Beltran-Leal A., Muñoz-Ruiz A., Esparza-Villalpando V., Castro Y., Pozos-Guillen A., Flores H. 5-Aminolevulinic acid photoactivated over planktonic and biofilm forms of Enterococcus faecalis as a pharmacological therapy alternative. Brazilian J Pharm Sci. 2020; 56: 1-9.
19. Kim R.J.Y., Kim M.O., Lee K.S., Lee D.Y., Shin JH. An in vitro evaluation of the antibacterial properties of three mineral trioxide aggregate (MTA) against five oral bacteria. Arch Oral Biol. 2015; 60 (10): 1497-502.
20. Al-Hezaimi K., Al-Shalan T.A., Naghshbandi J., Oglesby S., Simon J.H.S., Rotstein I. Antibacterial Effect of Two Mineral Trioxide Aggregate (MTA) Preparations Against Enterococcus faecalis and Streptococcus sanguis In Vitro. J Endod. 2006; 32 (11): 1053-6.
21. Chopra M.S., Gulve M.N. Evaluation of the Antibacterial and Antifungal Activity of Three Retrograde Filling Materials : An In Vitro Study. Int J Contemp Med Res. 2016; 3 (8): 2286-8.
22. Khedmat S., Aminipor M., Pourhajibagher M., Kharazifar M.J., Bahador A. Comparison of Antibacterial Activities of ProRoot MTA, OrthoMTA, and RetroMTA Against Three Anaerobic Endodontic Bacteria. J Dent. 2018; 15 (5): 294-9.
23. Demiryürek E.Ö., Özyürek T., Gülhan T. Evaluation of antibacterial and antifungal activity of calcium silicate based retrograde filling materials. Int J Appl Dent Sci. 2016; 2 (2): 85-8.
24. George S., Kishen A., Song K.P. The role of environmental changes on monospecies biofilm formation on root canal wall by Enterococcus faecalis. J Endod. 2005; 31 (12): 867-72.
25. Al-Hezaimi K., Naghshbandi J., Oglesby S., Simon J.H.S., Rotstein I. Comparison of antifungal activity of white-colored and gray-colored mineral trioxide aggregate (MTA) at similar concentrations against Candida albicans. J Endod. 2006; 32 (4): 365-7.
 Odovtos -Int J Dent Sc endoses to CC-BY-NC-SA 4.0.
Odovtos -Int J Dent Sc endoses to CC-BY-NC-SA 4.0.