Odovtos-International Journal of Dental Sciences (Odovtos-Int. J. Dent. Sc.), Online First, 2025. ISSN: 2215-3411
https://doi.org/10.15517/gzn6m691

https://revistas.ucr.ac.cr/index.php/Odontos
BASIC RESEARCH:
Antimicrobial Efficacy of Smear Clear in the Elimination of Enterococcus faecalis. An In Vitro Study
Eficacia antimicrobiana de smear clear en la eliminación de Enterococcus faecalis. Un estudio in vitro
Javier Alvear-Pérez¹ https://orcid.org/0000-0001-9748-7238
Stella Pupo-Marrugo¹ https://orcid.org/0000-0001-5897-0542
José Florez-Ariza² https://orcid.org/0000-0002-8788-5125
Liz Guerrero-Rodríguez3 https://orcid.org/0009-0004-6853-8867
Edgardo Montesino-Ortiz3 https://orcid.org/0009-0005-0285-600X
Jaime Plazas-Román⁴ https://orcid.org/0000-0002-5040-6899
¹Dentist, Universidad de Cartagena. Specialist in Endodontics, Professor, School of Dentistry, Universidad de Cartagena, Colombia.
²Dentist, Universidad de Cartagena. MSc in Oral and Maxillofacial Radiology. Specialist in Endodontics, Professor, School of Dentistry, Universidad de Cartagena, Colombia.
3Dentist, Universidad de Cartagena. Specialist in Endodontics, Universidad de Cartagena, Colombia.
⁴Dentist, Universidad de Cartagena. MSc in Bioinformatics, Professor, Universidad del Sinú Elías Bechara Zainúm, Seccional Cartagena, Professor, School of Dentistry, Universidad de Cartagena, Colombia.
Correspondence to: Jaime Plazas-Román - jplazasr@unicartagena.edu.co
Received: 7-V-2025 Accepted: 10-IX-2025
ABSTRACT: Microorganisms are fundamental etiological factors in pulpal and periapical diseases. Enterococcus faecalis, a Gram-positive facultative anaerobic coccus, can penetrate dentinal tubules and survive chemical-mechanical instrumentation. Endodontic success requires effective irrigation to reach areas inaccessible to instruments. Despite sodium hypochlorite's antimicrobial efficacy, its toxicity necessitates exploring alternatives. To evaluate Smear Clear®'s antimicrobial efficacy against E. faecalis. This in vitro study tested Smear Clear® against E. faecalis (ATCC 29212®) strain, determining its antimicrobial efficacy and establishing minimum inhibitory and bactericidal concentrations. Bacterial sensitivity was tested using microplate dilution techniques with appropriate controls. Smear Clear® at 100% showed bactericidal activity against E. faecalis. At 50% concentration, it demonstrated the minimum concentration needed for growth inhibition with bacteriostatic properties. Smear Clear® exhibits antimicrobial properties against E. faecalis and represents a potential alternative to sodium hypochlorite in endodontic irrigation, with its components, particularly cetrimide, directly affecting bacterial sensitivity.
KEYWORDS: Enterococcus faecalis; Root canal irrigants; Anti-bacterial agents; Microbial sensitivity tests; Biofilms; Dental pulp cavity.
RESUMEN: Los microorganismos son factores etiológicos fundamentales en enfermedades pulpares y periapicales. Enterococcus faecalis, un coco Gram-positivo anaerobio facultativo, puede penetrar túbulos dentinarios y sobrevivir a la instrumentación químico-mecánica. El éxito endodóntico requiere irrigación efectiva para alcanzar áreas inaccesibles a los instrumentos. A pesar de la eficacia antimicrobiana del hipoclorito de sodio, su toxicidad requiere explorar alternativas. Evaluar la eficacia antimicrobiana de Smear Clear® contra E. faecalis. Este estudio in vitro evaluó Smear Clear® contra la cepa E. faecalis (ATCC 29212®), determinando su eficacia antimicrobiana y estableciendo concentraciones mínimas inhibitorias y bactericidas mediante técnicas de microdilución en microplacas con controles apropiados. Smear Clear® al 100% mostró actividad bactericida contra E. faecalis. Al 50% demostró ser la concentración mínima necesaria para inhibir el crecimiento con propiedades bacteriostáticas. Smear Clear® exhibe propiedades antimicrobianas contra E. faecalis y representa una alternativa potencial al hipoclorito de sodio en irrigación endodóntica, con componentes, particularmente cetrimida, que afectan directamente la sensibilidad bacteriana.
PALABRAS CLAVE: Enterococcus faecalis; Irrigantes del conducto radicular; Agentes antibacterianos; Pruebas de sensibilidad microbiana; Biopelículas; Cavidad pulpar dental.
INTRODUCTION
There has consistently been a demand to eliminate microorganisms that survive in the root canal system to avoid failures in endodontic treatment, which produce pain, inflammation, and other symptoms that restrict the quality of life of the patient and the success of restorative treatments. Periapical lesions contain a variety of bacteria, including Gram-negative anaerobic bacilli, Gram-positive anaerobic cocci, and facultative anaerobic Streptococcus (1). Among these microorganisms, Enterococcus faecalis (E. faecalis) has emerged as particularly significant in endodontic research. This Gram-positive coccus, facultative anaerobic, immobile, and non-sporulated, is considered a normal inhabitant of the human gastrointestinal tract, yet has long been of interest to researchers because it was recognized as the causative agent of persistent periapical infections (2,3).
The microbial ecology in cases of endodontic failure presents distinctive characteristics compared to primary infections. Multiple studies explain that the microbiota of dental organs with endodontic treatment failures differ significantly from that normally found in untreated tooth canals. While primary infections show diverse microbial communities, the ecosystem of microorganisms found in teeth with failure in endodontic treatment is predominantly facultative anaerobic and Gram-positive, with E. faecalis being the species most frequently isolated. This prevalence can be attributed to its remarkable characteristic to survive and grow in microenvironments that could be toxic and vulnerable for many bacteria, in specific areas with high concentrations of salts and extreme temperatures (15-60ºC)(3).
The persistence of E. faecalis in the root canal system stems from its high virulence and adaptability to hostile environments. It is pertinent to highlight these characteristics which allow this microorganism to generate failures in endodontic therapy because once it penetrates into the root canal, it can adhere to the collagen of dentin, bone collagen, and other tissues, enter the dentinal tubules, resist drugs such as calcium hydroxide, survive irrigation with sodium hypochlorite, and form biofilm both at the intraradicular and extraradicular levels. These properties collectively favor the appearance of persistent periapical lesions, creating significant challenges for conventional endodontic treatment approaches (4).
Effective endodontic therapy requires comprehensive disinfection strategies targeting resistant microorganisms. To eliminate infection from the root canal system, it is necessary to clean and shape them, as well as to seal three-dimensionally, eliminating empty spaces that may have the potential to be infected or reinfected. Despite advances in irrigation solutions, there is no substance that has all the characteristics of an ideal irrigator of the root canal systems. The limitations of current irrigants have driven the search for alternatives with improved properties. Among the irrigants introduced, Smear Clear® (SybronEndo, Orange, CA, USA), which has been in use since at least 2006, has been studied as a potential solution (5,6). The formula contains 17% EDTA, cetrimide, and a special surfactant, combining chelating properties with antimicrobial potential. It is stated that the surface tension reducer improves the contact angle of the solution when placed on the dentin surface and enhances cleaning efficiency (5). Furthermore, research has demonstrated that the addition of cetrimide in irrigating solutions increased its antibacterial effects on Enterococcus faecalis in the dentinal tubules (6).
The scientific significance of this research lies in addressing a critical gap in endodontic antimicrobial strategies. Despite extensive knowledge of mechanical preparation and irrigation protocols, persistent infections caused by resistant microorganisms like E. faecalis continue to challenge treatment outcomes. While sodium hypochlorite remains the gold standard for canal disinfection, its cytotoxicity creates risks for periapical tissues. Finding alternative irrigants that combine effective cleaning with potent antimicrobial properties against resistant organisms would represent a significant advancement in clinical practice. Therefore, the objective of this investigation was to evaluate the antimicrobial efficacy of Smear Clear® in the elimination of Enterococcus faecalis, potentially establishing it as a multifunctional irrigant with improved safety profile for comprehensive endodontic disinfection.
MATERIALS AND METHODS
An in vitro experimental study was conducted, in which the antimicrobial activity of Smear Clear® was determined in the elimination of Enterococcus faecalis (ATCC 29212®).
Population and sample. The study evaluated the antibacterial activity of Smear Clear® (SybronEndo, Orange, CA, USA) against the reference strain of Enterococcus faecalis (ATCC 29212®), which is recommended for sensitivity and quality control tests according to CLSI(7), provided by the Molecular Biology and Genetics Group, School of Medicine of the University of Cartagena.
Preparation of culture medium. For the test, Mueller Hinton Agar was used as the culture medium due to its standardized composition for antimicrobial susceptibility testing, reliability in supporting the growth of non-fastidious microorganisms, and consistency with established protocols for evaluating antimicrobial efficacy. The medium was prepared according to the manufacturer's instructions (8). All procedures were performed inside a laminar flow cabinet to preserve the sterility of the culture medium.
It is important to highlight that, for sterility control of the medium, after preparation, it was sterilized in an autoclave at a temperature of 121˚C for 15 minutes and kept in a water bath until reaching a temperature between 48-50°C.
Microbiological methods. The Enterococcus faecalis strain (ATCC 29212®) was used, recommended for sensitivity testing. The strains of Enterococcus were obtained using the KWIK-STIK system, consisting of a swab containing the bacteria in a lyophilized state. That same system has a moisturizing liquid, which suspends the bacteria before being seeded in the culture medium. Once the bacteria grew, a purity control was carried out by performing a Gram stain, which showed Gram-positive cocci compatible with E. faecalis.
Preparation of the bacterial inoculum. Bacteria stored in freezing (-80ºC) were cultivated by scraping with a microbiological handle on the bacterial solution contained in thioglycolate plus glycerol and seeding them on nutritive agar using the depletion method. Subsequently, they were incubated within 15 minutes at 37°C in aerobiosis for 24 hours. After this time, approximately 5 to 10 colonies were taken and suspended in 5 milliliters (ml) of Mueller Hinton broth, shaking it in a vortex to achieve homogeneity of the suspension.
Preparation of work solutions at different concentrations. The Smear Clear® irrigant was taken starting from a concentration of 100%, from which dilutions were made with sterile distilled water to reduce its concentration, making decreasing dilutions to 50%, 25%, 12.5%, and 6.25% of the product.
Preparation of 5% sodium hypochlorite control. 5% sodium hypochlorite (Proquident) was also taken as a positive control due to its established antibacterial efficacy; 150 μL of 5% sodium hypochlorite and 150 μL of inoculum were added to the 96-well microplate in triplicate.
Growth curve. 5 to 10 colonies isolated from the bacterial culture were taken and inoculated in 5 ml Mueller Hinton broth and incubated for 1 hour at 37°C. 100 μL of the inoculum was taken and placed in a 96-well microplate, in triplicate for later reading in a MultiScan Ex reader (Thermo Scientific) on a scale of 0.08 - 0.1 absorbance, at a wavelength of 620 nanometers. Subsequently, a dilution of the inoculum was made in the Muller Hinton broth medium (in a ratio of 1-100). Readings were taken for 24 hours every 2 hours, with the first reading called zero hour, until reaching the maximum growth of the bacteria and its subsequent stationary state and death of the bacteria.
Evaluation of bacterial sensitivity. Once the preliminary tests were completed, the Smear Clear® solution at 100% was taken to evaluate bacterial sensitivity. For this, 150 μL of each concentration of Smear Clear® was added to a 96-well microplate (in triplicate) along with 150 μL of inoculum, with a concentration of approximately 1.5x10^8^ colony-forming units (CFU)/mL of the strain. The microplate was incubated at 37°C for 24 hours, which was the time indicated by the growth curve, and its density was determined in a MultiScan EX reader at a scale of 0.08 - 0.1 absorbance, at a wavelength of 620 nm. For the test, positive controls (300 μl of the inoculum), negative controls (150 μl of the inoculum + 150 μl of sodium hypochlorite), and sterility controls (300 μl of sterile broth without inoculation) were included.
Minimum inhibitory concentration (MIC). The minimum inhibitory concentration was determined by the microdilution technique in 96-well plates. For this, the Smear Clear® solution was serially diluted until reaching the solution with the lowest concentration in the bacterial suspension at a concentration of 5 x 10^5^ CFU/mL. As a negative control, 5% sodium hypochlorite was used, along with sterility controls using culture broth without inoculum and inoculum as a positive control. The plates were incubated at 37°C under anaerobic conditions for 24 hours. Using a Multiskan Thermo spectrophotometer (Multiskan EX, Thermo Scientific) with a wavelength at 620nm, changes in the optical density of the samples were evaluated. The lower concentration of the Smear Clear® irrigant that significantly inhibits bacterial growth was considered as the MIC.
Minimum bactericidal concentration (MBC). From the detected MIC, 10μl of bacterial suspension was added to sterile Petri dishes. These were incubated under the same conditions mentioned above for 24 hours. Subsequently, those concentrations where bacterial colony formation was present were visually identified. If the number of colonies was reduced, it was considered bacteriostatic activity. If, on the other hand, colonies did not form, bactericidal activity was assigned.
Statistical analysis
The results obtained in the evaluation of the sensitivity test curves and minimum inhibitory concentration were analyzed and plotted using Graphpad Prism 5.01 software, where a one-way analysis of variance (ANOVA) was carried out followed by the Dunnet post-test for multiple comparisons and considering a value of P<0.05 (95% confidence). The data was plotted as the mean and its standard deviation. Prior to the analysis, Kolmogórov-Smirnov's normality test was performed, using the Statistical Package for the Social Sciences (SPSS) version 20 software (IBM®).
RESULTS
The study began with the growth curve of E. faecalis (ATCC 29212®), where the behavior of the bacteria in the incubation period was observed. The adaptation period of the bacteria was approximately 4 hours, where the exponential phase of growth began, reaching the maximum peak at 12 hours. From this point, the stationary phase started until the 18-hour mark and subsequent bacterial death.
With this result, it was possible to determine the incubation time for the measurement of the bacterial sensitivity tests and determination of the minimum inhibitory concentration, which was performed after 12 hours.
Bacterial sensitivity. The sensitivity test was performed with the Smear Clear® solution at 100% concentration as presented by the manufacturer. The assay included four experimental conditions:
1. Positive growth control (Broth + E. faecalis).
2. Test condition (Broth+E. faecalis+Smear Clear® 100%).
3. Negative control (Broth+E. faecalis+sodium hypochlorite)
4. Sterility control (Broth only).
The results demonstrated that Smear Clear® at 100% concentration possesses significant antimicrobial activity against E. faecalis (ATCC 29212®). There was a statistically significant reduction in bacterial growth compared to the positive control (p<0.001), while no significant difference was observed when compared to the negative control (sodium hypochlorite) (p>0.05). This indicates that Smear Clear® exhibits antimicrobial efficacy comparable to sodium hypochlorite. These results validated the progression to subsequent testing of Minimum Inhibitory Concentration at dilutions of 50%, 25%, 12.5%, and 6.25%, as well as Minimum Bactericidal Concentration determination. The results of the bacterial sensitivity test are shown in Figure 1.
Evaluation of the minimum inhibitory concentration. In the findings of the minimum inhibitory concentration, it was observed that Smear Clear® at 50% had no significant difference compared to the broth; and that at concentrations of 25%, 12.5%, and 6.25%, there was a difference (p<0.05). With this information, it can be inferred that the minimum concentration needed of Smear Clear® to produce growth inhibition of E. faecalis (ATCC 29212®) is 50%. Figure 2 illustrates the minimum inhibitory concentration results at different Smear Clear® dilutions.
Minimum bactericidal concentration. Taking into account the evaluation of the minimum inhibitory concentration, those concentrations that did not obtain a statistically significant difference were chosen; that is, Smear Clear® concentrations at 100% and 50%, obtaining that the Smear Clear® irrigating solution has bactericidal properties on E. faecalis (ATCC 29212®) at 100% concentration and bacteriostatic properties at 50% concentration. The bactericidal effect of Smear Clear® at 100% concentration is demonstrated in Figure 3.
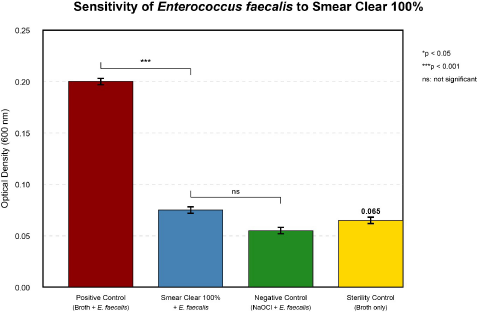
Figure 1. Antibacterial activity of Smear Clear® against Enterococcus faecalis (ATCC 29212®). Optical density measurements at 600 nm after 24h incubation. Values represent mean±SD (n=3). Statistical analysis: one-way ANOVA with Tukey's post-hoc test. ***p<0.001 vs positive control; ns: not significant vs negative control.
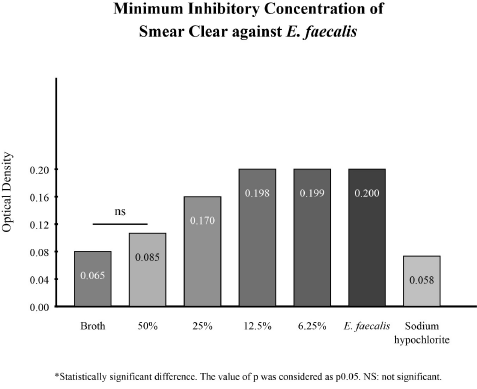
Figure 2. Minimum inhibitory concentration of Smear Clear® on Enterococcus faecalis (ATCC 29212®).

Figure 3. Minimum Bactericidal Concentration (MBC) of Smear Clear® at 100% concentration.
DISCUSSION
For the development of the present assay, the E. faecalis strain (ATCC 29212®) was used, which is the recommended strain for performing sensitivity and quality control tests according to CLSI, similar to that reported by Farac RV et al. (2013) in an ex vivo study on intracanal medication for contamination with Enterococcus faecalis (9). This methodological approach was further reinforced by Cuellar et al. (2017), who achieved comparable results using the same bacterial strain when evaluating bacterial extrusion in root canal retreatments with and without passive ultrasonic irrigation(10).
Although Smear Clear® has well-documented clinical applications as reported by Giardino L et al., there remains a significant research gap regarding its evaluation from a microbiological perspective. This knowledge deficit is particularly significant because bacterial contamination is widely established as the primary etiological factor in pulpal and periapical pathologies (11). Therefore, our investigation addresses a crucial need in endodontic research by providing objective conclusions on Smear Clear®'s antibacterial efficacy against E. faecalis, a gastrointestinal microorganism frequently associated with persistent endodontic infections, an area previously unexplored in the scientific literature.
In assessing irrigant efficacy, laboratory tests constitute essential initial investigations. Our bacteriological analysis employed the antimicrobial sensitivity technique via microplate dilution method with comprehensive controls, including sterility controls for medium or broth, positive controls for growth inhibition using sodium hypochlorite, and bacterial inoculum controls. This methodological rigor mirrors the approach used by Pimentel Ramírez et al. (2015), who similarly evaluated minimum inhibitory and bactericidal concentrations of plant extracts against oral bacteria like Lactobacillus acidophilus and Porphyromonas gingivalis (12). While we acknowledge the limitation of testing only one E. faecalis strain, this selection was strategically justified by the organism's documented high prevalence in endodontic infections, its established role in treatment failures, and its remarkable capacity to form treatment-resistant biofilms, characteristics extensively validated by Haapasalo and Shen in their research (13).
The relationship between antimicrobial efficacy and pH conditions provides an important context for our findings. MacHugh et al. demonstrated in their in vitro study that while pH 10.5-11.0 merely delays E. faecalis growth, environments at pH 11.5 or higher exhibit definitive bactericidal properties (14). Although our study did not explicitly include pH as a variable, our methodology allowed for evaluation of E. faecalis (ATCC 29212®) resistance under alkaline conditions. Notably, Smear Clear®'s composition begins with the neutral pH (7.5) typical of chelating agents but likely increases in alkalinity due to the addition of cetrimide and surfactant components, potentially contributing to its observed bactericidal effects through this pH-increasing mechanism.
Our findings regarding minimum inhibitory concentration align with other research examining antimicrobial agents against E. faecalis. Mojica D and Román Y's experimental work with plant extracts against vancomycin-resistant E. faecalis demonstrated effective inhibition at specific concentrations, employing a CLSI-based microdilution method similar to our protocol (15). Comparably, our evaluation determined that Smear Clear® solutions at both 100% and 50% concentrations showed no statistically significant differences in their ability to inhibit E. faecalis (ATCC 29212®) growth, establishing 50% as the minimum effective concentration. This finding is methodologically consistent with Herrera et al. (2014), who used similar microdilution techniques to establish the MIC and MBC values for Mammea americana against oral pathogens(16).
The synergistic effect of combined antimicrobial agents observed in our study finds significant parallels in existing literature. Ferrer C et al. demonstrated that while maleic acid alone effectively eradicated E. faecalis at 0.88% concentration within 30 seconds, combinations of 0.2% cetrimide with either 15% EDTA or 15% citric acid achieved complete bacterial destruction within just 1 minute of contact(17). These findings directly complement our results with Smear Clear®, which contains a similar combination of EDTA (15%) and cetrimide (0.75%). This compositional parallel explains the observed antimicrobial effectiveness and suggests that the mechanism of action involves both chelating properties and surfactant-enhanced antimicrobial activity, providing a comprehensive approach to endodontic disinfection.
CONCLUSION
From this study, it is concluded that Smear Clear® is an irrigant solution that has antimicrobial activity in the elimination of Enterococcus faecalis, which is the microorganism mainly associated with endodontic failures. Its components, especially cetrimide, directly affect its sensitivity. However, while these results demonstrate its antimicrobial efficacy, Smear Clear® should be considered as an adjunct in root canal system disinfection rather than a complete alternative to sodium hypochlorite, as additional studies on its ability to dissolve organic tissue would be necessary to support such a conclusion.
It was observed that the Smear Clear® irrigation solution at 100% concentration had efficacy on the microbial activity of Enterococcus faecalis (ATCC 29212®). The minimum concentration of Smear Clear® to inhibit the growth of Enterococcus faecalis (ATCC 29212®) was 50%. Smear Clear® at 100% possesses bactericidal property on Enterococcus faecalis (ATCC 29212®).
AUTHOR CONTRIBUTION STATEMENT: Conceptualization and design: J.A.P., S.P.M.; Methodology and validation: J.A.P., S.P.M. and J.F.A.; Formal Analysis: J. Plazas-Román; Investigation and data collection: L.G.R. and E.M.O.; Resources: S.P.M.; Data analysis and interpretation: J.P.R.; Writing-original draft preparation: J.F.A., J.P.R.; Writing-review & editing: J.A.P. and J.F.A.; Supervision: J.A.P.; Project administration: J.A.P.
Funding Acquisition: Not applicable.
CONFLICT OF INTEREST: The authors have stated explicitly that there are no conflicts of interest in connection with this article.
ETHICS: The ethics committee approval with the reference number 20180525.
SOURCE OF FUNDING: Non Funding.
REFERENCES
1. Gong S.Q., Huang Z.B., Shi W., Ma B., Tay F.R., Zhou B. In vitro evaluation of antibacterial effect of AH Plus incorporated with quaternary ammonium epoxy silicate against Enterococcus faecalis. J Endod. 2014 Oct; 40 (10): 1611-5. doi: 10.1016/j.joen.2014.03.010
2. Alghamdi F., Shakir M. The Influence of Enterococcus faecalis as a Dental Root Canal Pathogen on Endodontic Treatment: A Systematic Review. Cureus. 2020 Mar 13; 12 (3). doi: 10.7759/cureus.7257
3. Zhang H., Shen Y., Ruse N.D., Haapasalo M. Antibacterial activity of endodontic sealers by modified direct contact test against Enterococcus faecalis biofilm. J Endod. 2009; 35: 1051-5. doi: 10.1016/j.joen.2009.04.022
4. Bhuva B., Patel S., Wilson R., Niazi S., Beighton D., Mannocci F. The effectiveness of passive ultrasonic irrigation on intraradicular Enterococcus faecalis biofilms in extracted single-rooted human teeth. Int Endod J. 2010 Mar; 43 (3): 241-50. doi: 10.1111/j.1365-2591.2009.01672.x
5. Kuruvilla A., Jaganath B.M., Krishnegowda S.C., Ramachandra P.K., Johns D.A., Abraham A. A comparative evaluation of smear layer removal by using edta, etidronic acid, and maleic acid as root canal irrigants: An in vitro scanning electron microscopic study. J Conserv Dent. 2015 May-Jun; 18 (3): 247-51. doi: 10.4103/0972-0707.157266
6. Sabu N., Thomas N.A., Thimmaiah C., Joseph A.P., Jobe J., Palose P.S. Comparative Evaluation of Chlorhexidine and Cetrimide as Irrigants in Necrotic Primary Teeth: An In vivo Study. J Pharm Bioallied Sci. 2022 Jul; 14 (Suppl 1). doi: 10.4103/jpbs.jpbs_753_21
7. Humphries R., Bobenchik A.M., Hindler J.A., Schuetz A.N. Overview of Changes to the Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing, M100, 31st Edition. J Clin Microbiol. 2021 Nov 18; 59 (12). doi: 10.1128/JCM.00213-21
8. Khan A., Miller W.R., Axell-House D., Munita J.M., Arias C.A. Antimicrobial Susceptibility Testing for Enterococci. J Clin Microbiol. 2022 Sep 21; 60 (9). doi: 10.1128/jcm.00843-21
9. García-Solache M., Rice L.B. The Enterococcus: a Model of Adaptability to Its Environment. Clin Microbiol Rev. 2019 Jan 30; 32 (2). doi: 10.1128/CMR.00058-18
10. Aydin U., Zer Y., Zorlu Golge M., Kirkgoz Karabulut E., Culha E., Karataslioglu E. Apical extrusion of Enterococcus faecalis in different canal geometries during the use of nickel titanium systems with different motion types. J Dent Sci. 2017 Mar; 12 (1): 1-6. doi: 10.1016/j.jds.2016.03.013
11. Cai C., Chen X., Li Y., Jiang Q. Advances in the Role of Sodium Hypochlorite Irrigant in Chemical Preparation of Root Canal Treatment. Biomed Res Int. 2023 Jan 13; 2023: 8858283. doi: 10.1155/2023/8858283
12. Courric E., Brinvilier D., Couderc P., Ponce-Mora A., Méril-Mamert V., Sylvestre M., Pelage J.H., Vaillant J., Rousteau A., Bejarano E., Cebrian-Torrejon G. Medicinal Plants and Plant-Based Remedies in Grande-Terre: An Ethnopharmacological Approach. Plants (Basel). 2023 Feb 2; 12 (3): 654. doi: 10.3390/plants12030654
13. Haapasalo M., Shen Y., Wang Z., Gao Y. Irrigation in endodontics. Br Dent J. 2014 Mar; 216 (6): 299-303. doi: 10.1038/sj.bdj.2014.204
14. Weckwerth P.H., Zapata R.O., Vivan R.R., Tanomaru Filho M., Maliza A.G., Duarte M.A. In vitro alkaline pH resistance of Enterococcus faecalis. Braz Dent J. 2013 Sep-Oct; 24 (5): 474-6. doi: 10.1590/0103-6440201301731
15. Hosseini S.H., Sadeghi Z., Hosseini S.V., Bussmann R.W. Ethnopharmacological study of medicinal plants in Sarvabad, Kurdistan province, Iran. J Ethnopharmacol. 2022 Apr 24; 288: 114985. doi: 10.1016/j.jep.2022.114985
16. Herrera Herrera A., Franco Ospina L., Fang L., Díaz Caballero A. Susceptibility of Porphyromonas gingivalis and Streptococcus mutans to Antibacterial Effect from Mammea americana. Adv Pharmacol Sci. 2014; 2014: 384815. doi: 10.1155/2014/384815
17. Ferrer-Luque C.M., Arias-Moliz M.T., González-Rodríguez M.P., Baca P. Antimicrobial activity of maleic acid and combinations of cetrimide with chelating agents against Enterococcus faecalis biofilm. J Endod. 2010 Oct; 36 (10): 1673-5. doi: 10.1016/j.joen.2010.06.009
 Odovtos -Int J Dent Sc endoses to CC-BY-NC-SA 4.0.
Odovtos -Int J Dent Sc endoses to CC-BY-NC-SA 4.0.