Odovtos-International Journal of Dental Sciences (Odovtos-Int. J. Dent. Sc.), Online First, 2025. ISSN: 2215-3411
https://doi.org/10.15517/92e4k728

https://revistas.ucr.ac.cr/index.php/Odontos
BASIC RESEARCH:
Effectiveness of Deproteinized Bovine Bone in Maxillary Sinus Bone Regeneration:
A Systematic Review and Descriptive Synthesis of Histological Evidence
Eficacia del hueso bovino desproteinizado en la regeneración ósea del seno maxilar:
una revisión sistemática y síntesis descriptiva de la evidencia histológica
Joaquín Juan Urbizo Vélez¹https://orcid.org/0000-0003-0387-0952
Alain Manuel Chaple Gil² https://orcid.org/0000-0002-8571-4429
Meylin Santiesteban-Velazquez3 https://orcid.org/0009-0008-5935-9313
¹Universidad de Ciencias Médicas de La Habana. Facultad de Estomatología “Raúl González Sánchez”. La Habana, Cuba.
²Universidad Autónoma de Chile. Facultad de Ciencias de la Salud. Santiago de Chile, Chile.
3Universidad de Ciencias Médicas de La Habana. ICBP “Victoria de Girón”. La Habana, Cuba.
Correspondence to: Alain Manuel Chaple Gil - alain.chaple@uautonoma.cl
Received: 20-II-2025 Accepted: 14-X-2025
ABSTRACT: Surgical maxillary sinus lifting for the placement of dental implants is a widely used procedure. A crucial step in these maneuvers is the placement of grafts to facilitate the rapid regeneration of bone tissue and achieving a base for implants osseointegration. To evaluate the histological and histomorphometric outcomes of maxillary sinus floor elevation procedures performed with deproteinized bovine bone, synthesizing the available evidence on its regenerative effectiveness. This systematic review, following PRISMA guidelines, analyzed studies from November 2024 to February 2025 on maxillary sinus floor elevation using deproteinized bovine bone. Using the PICO framework, it focused on histological bone regeneration outcomes. Searches in PubMed, Scopus, Web of Science, and grey literature were screened by two reviewers, with bias assessed using Cochrane’s RoB 2 and ROBINS-I tools. Fourteen studies were included. All RCTs and NRS showed low risk of bias. Bone formation ranged from 17.3% (Bio-Oss®) to 80.8% (SmartBone®). Combined grafts with BMAC or Emdogain® enhanced outcomes. Within the limitations of the current evidence, Bio-Oss and HA-TCP appear to be viable options for maxillary sinus bone regeneration, though differences exist in resorption rates and bone formation properties. Due to study heterogeneity, unequal group sizes, and risk of bias, the certainty of these findings is low, and further robust long-term trials are required.
KEYWORDS: Bone substitutes; Bone transplantation; Biomaterials; Hydroxyapatite; Tricalcium phosphate; Maxillary sinus; Bone regeneration.
RESUMEN: La elevación quirúrgica del seno maxilar para la colocación de implantes dentales es un procedimiento ampliamente utilizado. Un paso crucial en estas maniobras es la colocación de injertos para facilitar la rápida regeneración del tejido óseo y lograr una base para la osteointegración de los implantes. Evaluar los resultados histológicos e histomorfométricos de los procedimientos de elevación del seno maxilar realizados con hueso bovino desproteinizado, sintetizando la evidencia disponible sobre su eficacia regenerativa. Esta revisión sistemática, siguiendo las directrices PRISMA, analizó estudios publicados entre noviembre de 2024 y febrero de 2025 sobre la elevación del suelo del seno maxilar con hueso bovino desproteinizado. Aplicando el marco PICO, se enfocó en los resultados de regeneración ósea histológica. Las búsquedas en PubMed, Scopus, Web of Science y literatura gris fueron evaluadas por dos revisores, y el sesgo se valoró mediante las herramientas RoB 2 y ROBINS-I de Cochrane. Se incluyeron catorce estudios. Todos los ECA y ECNR mostraron bajo riesgo de sesgo. La formación ósea varió entre 17,3% (Bio-Oss®) y 80,8% (SmartBone®). Las combinaciones con BMAC o Emdogain® mejoraron los resultados. Dentro de las limitaciones de la evidencia actual, Bio-Oss y HA-TCP parecen ser opciones viables para la regeneración ósea del seno maxilar, aunque difieren en sus tasas de reabsorción y propiedades de formación ósea. Debido a la heterogeneidad de los estudios, el tamaño desigual de los grupos y el riesgo de sesgo, la certeza de estos hallazgos es baja y se requieren ensayos clínicos más robustos y de mayor seguimiento.
PALABRAS CLAVE: Sustitutos óseos; Trasplante óseo; Biomateriales; Hidroxiapatita; Fosfato tricálcico; Seno maxilar; Regeneración ósea.
INTRODUCTION
The effectiveness of deproteinized bovine bone (DBB) in maxillary sinus bone regeneration has been extensively studied, revealing promising outcomes in various clinical settings. Research indicates that DBB can facilitate significant bone augmentation, comparable to other grafting materials, while also demonstrating favorable histological characteristics.
A randomized clinical trial showed that sinus lift surgery with DBB resulted in an average bone augmentation of 10.31 mm, with a success rate of 96.3% (1). Another study found no significant differences in clinical outcomes between DBB and freeze-dried bone allograft (FDBA), although FDBA exhibited higher gene expression related to bone formation (2).
Histological analyses revealed that DBB particles were surrounded by immature woven bone, indicating effective integration and new bone formation (1). A systematic review indicated that both large and small granular DBB yielded similar histological results, suggesting that particle size does not significantly affect bone regeneration outcomes (3).
Studies comparing DBB with other materials, such as deproteinized porcine bone, found comparable new bone formation and graft stability (4). However, a study indicated that higher proportions of autogenous bone in combination with DBB led to better bone regeneration outcomes (5).
While DBB demonstrates effectiveness in maxillary sinus augmentation, some studies suggest that alternatives like Tricalcium phosphate-hydroxyapatite may offer superior biological responses, indicating a need for further research to optimize grafting strategies.
Given the growing interest in biological and synthetic graft materials, particularly deproteinized bovine bone and tricalcium phosphates, there is the need to up-to-date systematic reviews that consolidate existing evidence on their effectiveness in maxillary sinus augmentation. Several reviews have attempted to consolidate all available evidence on this subject. However, they mainly focused on the clinical findings and did not evaluate bone regeneration through histology and/or histomorphometry. It is essential to know the biological events that take place in cells and tissues in response to the influence of diverse biomaterials placed in the maxillary sinus cavities. This is only achieved through microscopical study. This study tries to fill the existing emptiness in this knowledge, to contribute to the improvement in the quality of treatment of this important issue.
Question
What is the effectiveness of deproteinized bovine bone in promoting maxillary sinus bone regeneration as evidenced by histological and histomorphometric findings in human studies?
Objective
To evaluate the histological and histomorphometric outcomes of maxillary sinus floor elevation procedures performed with deproteinized bovine bone, synthesizing the available evidence on its regenerative effectiveness.
METHODOLOGY
This systematic review followed the guidelines of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (6) to ensure that the research process was transparent, comprehensive, and reproducible. The protocol to develop this review was registered in PROSPERO with the ID: CRD42025648997.
The research project was developed in collaboration of “Universidad Autónoma de Chile” with “Universidad de Ciencias Médicas de La Habana” from November 2024 to February 2025.
In this systematic review, the PICO framework was applied to ensure the research question is specific, focused, and aligned with the objectives of the review. Below is an explanation of how each component of PICO was defined and applied within this study:
In this systematic review, the PICO framework was applied to ensure that the research question remained specific, focused, and consistent with the review’s objectives. The components were defined as follows:
P (Population): Human subjects who underwent surgical elevation of the maxillary sinus floor for bone regeneration purposes.
I (Intervention): Use of deproteinized bovine bone (DBB) as the primary grafting material.
C (Comparator): Studies without a direct comparator or those reporting descriptive outcomes of DBB performance, with secondary references to other graft materials when available.
O (Outcome): Histological and histomorphometric evidence of new bone formation, tissue integration, and graft resorption capacity.
Inclusion and exclusion criteria
The studies included in this systematic review were selected based on predefined criteria to ensure a comprehensive and unbiased assessment of the evidence. The inclusion criteria were as follows:
Population: Studies involving human subjects in which maxillary sinus floor elevation was performed for regenerative purposes.
Intervention: Use of deproteinized bovine bone (DBB) as the primary grafting material, either alone or in combination with biological or synthetic adjuncts.
Comparator: Not required. However, studies reporting comparative or descriptive histological outcomes involving DBB and other grafting materials were included when relevant.
Outcome Measures: Studies that evaluated bone regeneration through histological and/or histomorphometric analysis, including parameters such as new bone formation, graft resorption, and tissue integration.
Study Design: Randomized controlled trials (RCTs), non-randomized clinical studies (NRS), prospective or retrospective observational studies, and human case series reporting histological outcomes.
Literature Type: Peer-reviewed journal articles, theses, dissertations, conference proceedings, and technical reports were included to account for grey literature sources and reduce publication bias.
Exclusion criteria were applied to filter out studies that could introduce bias or were not directly relevant to the review’s objectives:
- Studies involving animal or in vitro models.
- Studies that did not involve the maxillary sinus or did not focus on bone regeneration.
- Reviews, meta-analyses, editorials, opinion papers, and letters to the editor.
- Studies lacking quantitative or descriptive histological or histomorphometric data.
- Studies where deproteinized bovine bone was combined with unrelated or experimental materials not aimed at bone regeneration.
- Duplicated publications, incomplete reports, or studies with unavailable full texts.
By incorporating grey literature sources such as theses, conference abstracts, and technical reports, this review aimed to minimize publication bias and to capture potentially valuable but unpublished histological data.
Search strategy
A comprehensive search strategy was implemented to maximize the identification of relevant literature. The search was conducted across three databases (PubMed, Scopus, and Web of Science), which provided broad coverage all disciplines. The search terms combined controlled vocabulary (such as MeSH terms) and free-text keywords, ensuring that both formally indexed studies and those using emerging terminology were included. Boolean operators (AND, OR) and search modifiers were applied to refine the search and capture articles discussing the evaluation of the regenerative capacity of deproteinized bovine bone in the surgical elevation of the maxillary sinus floor, by experimental histological comparison with the use of hydroxyapatite-beta tricalcium phosphate, to determine its efficacy as bone graft material.
The complete electronic search strategies for PubMed, Scopus, and Web of Science are provided in Supplementary Table 1, including the full Boolean syntax, filters applied, and the number of records retrieved per database. All searches were conducted from January to February 2025. The search was not restricted by language or publication year, except where database filters were automatically applied (Table 1).
A search in grey literature was developed in: ProQuest Dissertations & Theses Global, Open Access Theses and Dissertations (OATD), Teseo, IEEE Xplore, arXiv, medRxiv, OpenGrey, National Technical Reports Library (NTRL), European Union Open Data Portal, Cochrane Library and ClinicalTrials.gov.
The search on these repositories was developed using the generic formulation below:
("bovine deproteinized bone" OR "deproteinized bovine bone" OR "Bio-Oss" OR "demineralized bovine bone") AND ("hydroxyapatite" OR "beta-tricalcium phosphate" OR "HA-β-TCP" OR "calcium phosphates") AND ("sinus floor elevation" OR "maxillary sinus augmentation" OR "sinus lift") AND ("bone regeneration" OR "bone healing" OR "osteogenesis" OR "bone repair") AND ("histological analysis" OR "histomorphometry" OR "histological evaluation").
The manuscripts resulting from the search for this grey literature were considered as "reports" and those derived from the databases were defined as "studies".
Table 1. Formulation employed in each database.
|
Database |
Formulation |
Filters |
|
Pubmed |
((("Bovine deproteinized bone" [Title/Abstract]) OR ("Deproteinized bovine bone matrix" [Title/Abstract]) OR ("Bovine bone graft" [Title/Abstract]) OR ("Bio-Oss" [Title/Abstract]) OR ("Demineralized bovine bone" [Title/Abstract])) AND (("Bone regeneration" [Title/Abstract]) OR ("Bone formation" [Title/Abstract]) OR ("Bone healing" [Title/Abstract]) OR ("Osteogenesis" [Title/Abstract]) OR ("Bone repair"[Title/Abstract])) AND (("Sinus floor elevation" [Title/Abstract]) OR ("Maxillary sinus augmentation" [Title/Abstract]) OR ("Sinus lift" [Title/Abstract]) OR ("Sinus grafting procedure" [Title/Abstract]) OR ("Maxillary sinus floor augmentation" [Title/Abstract])) AND (("Histological analysis" [Title/Abstract]) OR ("Histology of bone regeneration" [Title/Abstract]) OR ("Histomorphometric analysis" [Title/Abstract]) OR ("Histological evaluation" [Title/Abstract]) OR ("Tissue engineering histology" [Title/Abstract]))) OR ((("Bone Substitutes" [MeSH Terms]) OR ("Bone Transplantation" [MeSH Terms]) OR ("Biomaterials" [MeSH Terms]) OR ("Xenograft" [MeSH Terms])) AND (("Bone Regeneration" [MeSH Terms]) OR ("Bone Remodeling" [MeSH Terms]) OR ("Osteogenesis" [MeSH Terms]) OR ("Wound Healing" [MeSH Terms])) AND (("Maxillary Sinus" [MeSH Terms]) OR ("Sinus Floor Augmentation" [MeSH Terms]) OR ("Oral Surgical Procedures" [MeSH Terms])) AND (("Histological Techniques" [MeSH Terms]) OR ("Histology" [MeSH Terms]) OR ("Histomorphometry" [MeSH Terms]) OR ("Tissue Engineering" [MeSH Terms])))) |
Filters applied: Clinical Study, Clinical Trial, Randomized Controlled Trial. |
|
Scopus |
TITLE-ABS-KEY(("bovine deproteinized bone" OR "deproteinized bovine bone matrix" OR "bovine bone graft" OR "Bio-Oss" OR "demineralized bovine bone") AND ("bone regeneration" OR "bone formation" OR "bone healing" OR "osteogenesis" OR "bone repair") AND ("sinus floor elevation" OR "maxillary sinus augmentation" OR "sinus lift" OR "sinus grafting procedure" OR "maxillary sinus floor augmentation") AND ("histological analysis" OR "histology of bone regeneration" OR "histomorphometric analysis" OR "histological evaluation" OR "tissue engineering histology")) AND (KEY("bone substitutes" OR "hydroxyapatites" OR "tricalcium phosphate" OR "biocompatible materials" OR "maxillary sinus" OR "bone regeneration")) |
Filters were not applied. |
|
WoS |
TS=("bovine deproteinized bone" OR "deproteinized bovine bone matrix" OR "bovine bone graft" OR "Bio-Oss" OR "demineralized bovine bone") AND TS=("bone regeneration" OR "bone formation" OR "bone healing" OR "osteogenesis" OR "bone repair") AND TS=("sinus floor elevation" OR "maxillary sinus augmentation" OR "sinus lift" OR "sinus grafting procedure" OR "maxillary sinus floor augmentation") AND TS=("histological analysis" OR "histology of bone regeneration" OR "histomorphometric analysis" OR "histological evaluation" OR "tissue engineering histology") AND TS=("bone substitutes" OR "hydroxyapatites" OR "tricalcium phosphate" OR "biocompatible materials" OR "maxillary sinus" OR "bone regeneration") |
Filters were not applied. |
Study Selection Process
The study selection process involved a two-stage screening to minimize bias. First, titles and abstracts were screened by two independent reviewers to exclude irrelevant studies. Next, the full texts of selected studies were assessed against the eligibility criteria. Any disagreements between the reviewers were resolved through discussion or consultation with a third reviewer if necessary. This rigorous screening process ensured consistency and prevented the inclusion of irrelevant or low-quality studies. The PRISMA flow diagram was used to document each stage of the screening process, providing a clear visual representation of how studies were identified, screened, and included or excluded.
A calibration was performed among the authors to evaluate the articles to be selected. The degree of coincidence of the evaluations made by the reviewers was determined using Orwin's method of 1994, and a Kappa statistic was performed to measure the agreement among the reviewers who would make simple decisions about inclusion/exclusion. Kappa values between 0.40 and 0.59 were considered to reflect acceptable agreement, 0.60 to 0.74 to be an adequate agreement, and 0.75 or more to reflect excellent agreement.
Risk of Bias Assessment
In this systematic review, the risk of bias assessment of (RCTs was conducted with the Cochrane's Risk of Bias 2 (RoB 2)(7), from which studies are classified as high, low, no information, or “some concerns” risk of bias, considering the domains related to the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported results. For non-randomized studies, the Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) (8) tool was employed, which evaluates seven domains including confounding, selection of participants, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of the reported results. The risk of bias for all types of studies was independently assessed by two reviewers, and any disagreements were resolved through discussion.
Data Extraction
Data extraction was performed systematically using a predesigned data extraction form to ensure consistency and accuracy across all included studies. The following key variables were extracted for further processing and analysis:
Study Identification: Title, authors, year of publication, journal or source, country of study, and study design.
Population Characteristics: Sample size, demographic details of the subjects, and the type of model.
Intervention Details: Type of grafting material used (deproteinized bovine bone or hydroxyapatite-beta tricalcium phosphate), dosage, concentration, or any specific preparation protocols applied.
Comparator Details: Information on the comparator material (if applicable), including its composition and method of application.
Procedure Details: Surgical approach for the maxillary sinus floor elevation, adjunctive therapies, duration of follow-up, and any reported intraoperative or postoperative complications.
Outcome Measures: The primary variable analyzed across all studies was histomorphometric bone formation, defined as the percentage of newly formed bone relative to the total tissue area within the grafted region. This quantitative histomorphometric measure served as the main indicator of bone regeneration, enabling standardized comparison among studies. Secondary outcomes included qualitative histological parameters of bone quality (e.g., vascularization, tissue integration, residual graft presence) and any relevant biological or inflammatory markers reported.
Data Reporting: Means, standard deviations, confidence intervals, or any statistical measures provided for bone regeneration outcomes.
Study Quality and Risk of Bias Indicators: Information on randomization, blinding, allocation concealment, and loss to follow-up, if reported.
Additionally, any relevant data from grey literature sources (e.g., unpublished theses or technical reports) were carefully extracted to maintain consistency with peer-reviewed studies. Missing data or unclear information were documented, and attempts were made to contact corresponding authors for clarification when necessary.
These extracted variables were chosen to support both qualitative synthesis and potential quantitative analysis, with the histomorphometric percentage of new bone formation considered the principal outcome for comparing the regenerative capacity of deproteinized bovine bone and hydroxyapatite-beta tricalcium phosphate in maxillary sinus floor elevation.
Data Analysis
Given the heterogeneity in grafting materials, particle sizes, follow-up times, and outcome measurement techniques, a formal meta-analysis was not performed.
The retrieved articles were organized in an Excel spreadsheet and processed using RStudio® 2024.12.0 Build 467.
All data extracted from the included articles, data processing and screening is available in data repository: https://doi.org/10.17632/gzths75pnz.1
RESULTS
No reports from the gray literature were identified for inclusion in the present study; therefore, the review focused solely on results obtained from PubMed, WoS, and Scopus.
Study Selection Process
The study selection process, summarized in Figure 1, followed a comprehensive and systematic approach to ensure the inclusion of relevant studies for analysis. Initially, 169 records were identified from three electronic databases: PubMed (n=49), Scopus (n=56), and Web of Science (WoS) (n=64). After the removal of 30 duplicate records, 139 unique studies proceeded to the screening phase.
During screening, the abstracts and titles of these records were assessed for relevance, leading to the exclusion of 122 studies that did not meet the inclusion criteria. This step left 17 studies eligible for full-text assessment. No studies were marked as “sought for retrieval” or excluded due to unavailability, as all identified reports were successfully retrieved.
Among the 17 reports assessed for eligibility, one study was excluded because it did not relate to the scope of research, as specified in the predefined eligibility criteria, other the assessment of bone height not suitable for inclusion in the study and a last one that no use of bovine bone. Consequently, 14 studies (1, 9-21) were included in the final qualitative synthesis.
This rigorous selection process aimed to enhance the reliability and relevance of the findings by including only studies that met the established inclusion criteria. The detailed flow of records through the selection stages provides transparency and replicability for future systematic reviews.
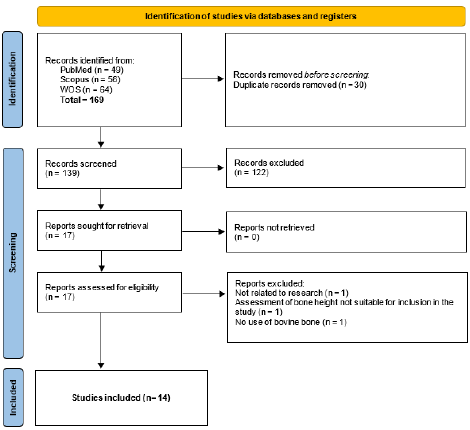
Figure 1. Flowchart of study selection process.
Risk of Bias Assessment
The evaluation of RCTs using the RoB 2 tool revealed that the majority of studies were judged to be at low risk of bias across most domains. Specifically, six out of seven trials demonstrated consistently low risk in domains related to randomization, deviations from intended interventions, outcome measurement, and selective reporting. The overall assessment indicated that all but one RCT were judged as low risk of bias (Figure 2.A).
The analysis of non-randomized studies using the ROBINS-I tool demonstrated a greater variability in risk of bias. Serious concerns were identified in two studies(9, 21), primarily due to confounding and outcome selection. Moderate risk was noted in domains such as participant selection, confounding, and reporting in several studies, including Kurkcu (2012), Lee (2012), Pasquali (2015), and Pignaton (2020). In contrast, Pereira (2024) presented low risk of bias across all domains. Overall, the non-randomized studies were mostly judged to be at low or moderate risk of bias, with only a few presenting higher concerns in specific domains. Consequently, these isolated cases are unlikely to substantially affect the overall reliability of the evidence base (Figure 2.B).
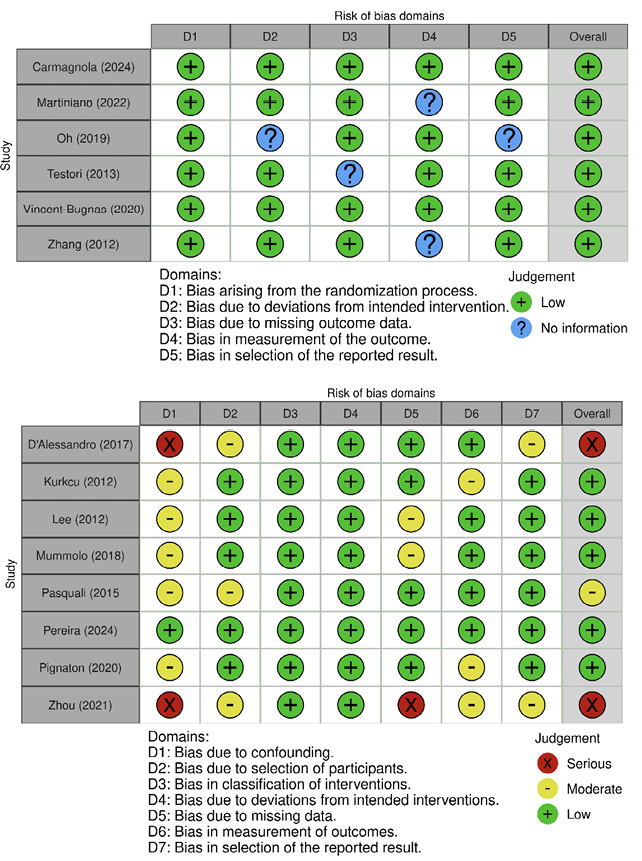
Figure 2. Risk of bias assessment of the included studies. (A) Risk of bias in randomized controlled trials (RoB 2 tool). Most trials showed low risk across the evaluated domains, with only one study presenting some concerns. (B) Risk of bias in non-randomized studies (ROBINS-I tool). A higher variability in bias was observed, with several studies judged as having moderate or serious risk, particularly due to confounding and outcome selection.
Results
The included studies, summarized in Table 2, evaluated different interventions for bone regeneration in sinus lift procedures, focusing on histomorphometric outcomes, bone formation percentages, and biomaterial performance. Overall, the results revealed significant differences between biomaterials and techniques, with several studies highlighting the impact of combined interventions on bone formation.
Among the included studies, both RCTs and NRSs were identified. A total of eight investigations were conducted as RCTs, whereas nine were designed as NRSs. This distribution demonstrated that although high-level evidence was available, a substantial proportion of the literature still relied on non-randomized designs to evaluate bone regeneration outcomes following maxillary sinus augmentation.
The included studies classified the primary biomaterial employed in maxillary sinus floor elevation according to its nature. Most of them reported the use of bovine-derived grafts, such as deproteinized bovine bone (Bio-Oss®, Cerabone®, ABB or Laddec), while a smaller number relied on synthetic substitutes, including biphasic calcium phosphate. This classification allowed the distinction between xenogeneic and synthetic materials, which is relevant for interpreting their osteoconductive potential, integration capacity, and degree of resorption.
Of the fourteen studies analyzed, all evaluated xenogeneic bovine bone grafts in different modalities, either used alone or combined with other biomaterials, assessing their comparative performance under varying conditions. In contrast, only two studies focused specifically on tricalcium phosphate as the main grafting material.
Regarding the mode of application, grafting materials were either used as the sole substitute or in combination with other biomaterials or biological adjuncts. Examples of combined approaches included the association of bovine bone with polymers, Bone Marrow Aspirate Concentrate (BMAC), Emdogain, or Platelet-Rich Fibrin (PRF). The distinction between single and combined use is clinically meaningful, since adjuvant strategies aim to potentiate vital bone formation and accelerate remodeling, whereas single-material applications allow for the evaluation of the intrinsic regenerative performance of the graft.
All included studies shared the use of histomorphometry as the primary analytical method, focusing on new bone formation as the main outcome variable. Bone formation was uniformly expressed as the percentage of newly formed bone relative to the total tissue area, ensuring comparability across studies despite methodological differences.
Carmagnola (1)(2024) evaluated a graftless technique against the use of deproteinized bovine bone (DBBP) and found comparable clinical outcomes and histological evidence of new bone formation in both cases. D’Alessandro (9) (2017) demonstrated high levels of bone formation, reaching 80.8% at six months, using SmartBone®, a biomaterial that underwent nearly complete resorption. Kurkcu (10)(2012) observed superior bone formation with bovine hydroxyapatite (30.13%) compared to beta-tricalcium phosphate (21.09%), emphasizing the potential of bovine-based materials.
Pereira (16)(2024) showed that Cerabone® led to significantly higher bone formation (25.94%) than Bio-Oss® (17.29%), reinforcing the efficacy of alternative materials. The benefits of combining biomaterials with growth factors were also evident. Pasquali (15)(2015) demonstrated that the addition of bone marrow aspirate concentrate (BMAC) to Bio-Oss significantly increased bone formation (55.15%) compared to Bio-Oss alone (27.3%). Similarly, Vincent-Bugnas (19)(2020) found that combining Bio-Oss with Emdogain® improved bone formation outcomes (22.6% versus 15.5% for Bio-Oss alone). Zhang (20)(2012) evaluated the effect of platelet-rich fibrin (PRF) combined with Bio-Oss in sinus lifts, showing a slight increase in bone formation (18.35%) compared to Bio-Oss alone (12.95%), although the difference was not statistically significant.
The outcomes of histomorphometric evaluations also varied based on particle size and anatomical factors. Testori (18)(2013) found that larger particles of anorganic bovine bone mineral (ABBM) promoted greater bone formation (26.77%) compared to smaller particles (18.77%), suggesting that particle size influences the regenerative potential. Zhou (21)(2021) highlighted the role of sinus anatomy in bone regeneration, reporting that wider sinuses were associated with reduced new bone formation (18.25%) due to a negative correlation between sinus width and bone growth.
Table 2. Summary of included studies and primary outcomes.
|
Author (year) |
Sample size |
Used alone or combined |
Study type |
Intervention or exposure |
Primary outcome (s) |
|
Carmagnola (1) (2024) |
19 |
Alone |
RCT |
Sinus lift with DBBP (control) vs graftless technique (test) |
Similar clinical results between grafted and non-grafted techniques. Histology showed a new bone. |
|
D'Alessandro (9 (2017) |
5 |
Combined |
NRS |
SmartBone® (bovine combined with biopolymers) |
High bone formation was observed (80.8% at 6 months) with almost total resorption of the material. |
|
Kurkcu (10 (2012) |
23 |
Alone |
NRS |
BHA (bovina) vs b-TCP (beta-tricalcium phosphate) |
Greater bone formation with BHA (30.13%) compared to b-TCP (21.09%). |
|
Lee (11) (2012) |
25 |
Alone |
NRS |
Sinus lift with Deproteinized Bovine Bone Mineral (DBBM) |
Successful sinuses lift with detailed histomorphometric results, good DBBM integration. |
|
Martiniano (12) (2022) |
10 |
Combined |
RCT |
Bio-Oss® vs Lumina-Porous® |
Bio-Oss showed a greater amount of vital bone compared to Lumina-Porous®. |
|
Mummolo (13) (2018) |
11 |
Alone |
NRS |
Sinus lift with Bio-Oss and Laddec (split-mouth) |
Both biomaterials showed adequate bone regeneration; Laddec showed greater absorbility than Bio-Oss. |
|
Oh (14) (2019) |
60 |
Combined |
RCT |
Biphasic calcium phosphate vs deproteinized bovine bone |
Both materials showed good osteoconductivity, although DBBM had higher residual volume. |
|
Pasquali (15) (2015 |
16 |
Combined |
NRS |
Bio-Oss combined with BMAC vs Bio-Oss only |
BMAC resulted in greater bone formation (55.15% vs 27.3%) and greater resorption of Bio-Oss compared to the control group. |
|
Pereira (16) (2024) |
22 |
Combined |
NRS |
Cerabone vs Bio-Oss |
Cerabone presented greater bone formation (25.94%) compared to Bio-Oss (17.29%), showing statistically significant differences. |
|
Pignaton (17) (2020) |
20 |
Alone |
NRS |
ABB (anorganic bovine bone) |
Evaluation of bone formation according to distance from native bone; the 1st mm showed more new bone (31.62%). |
|
Testori (18) (2013) |
13 |
Alon |
RCT |
ABBM (Bio-Oss) in large (1-2 mm) vs small (0.25-1 mm) particles |
Greater bone formation in large particles (26.77%) compared to small particles (18.77%). |
|
Vincent-Bugnas (19) (2020) |
16 |
Combined |
RCT |
Bio-Oss combined with Emdogain (test) vs Bio-Oss alone (control) |
Emdogain improved bone formation (22.6% compared to 15.5% in the control group). |
|
Zhang (20) (2012) |
11 |
Combined |
RCT |
Sinus lift with PRF + Bio-Oss vs Bio-Oss only. |
PRF showed a slight but not significant increase in new bone formation (18.35% vs 12.95%). |
|
Zhou (21) (2021) |
37 |
Alone |
NRS |
DBBM (Bio-Oss) with anatomical evaluation of the sinus cavity |
Wide sinuses showed less formation of new bone (18.25%), negative correlation with sinus width. |
NRS: Non-Randomized Studies DBBP: Deproteinized Bovine Bone Particles DBBM: Deproteinized Bovine Bone Mineral BHA: Bovine Hydroxyapatite b-TCP: Beta-Tricalcium Phosphate BMAC: Bone Marrow Aspirate Concentrate BBM: Anorganic Bovine Bone Mineral PRF: Platelet-Rich Fibrin.
DISCUSSION
The systematic review conducted, which included the most relevant studies, revealed no statistically significant differences in the effectiveness of the various biomaterials and techniques used for maxillary sinus bone regeneration. However, several studies emphasized the role of combined interventions in optimizing the regenerative process.
Most of the studies analyzed utilized xenogeneic grafts derived from bovine bone in different forms, evaluating variations in manufacturers, particle sizes, and porosity levels (11-13, 16, 18, 22). These materials were frequently combined with other biomaterials, (9, 15, 19, 20) hydroxyapatite-beta tricalcium phosphate (β-TCP),(10, 14) or additional factors (1, 17, 21, 23). Based on the data analyzed, all these materials demonstrated effectiveness in promoting bone tissue formation in the maxillary sinus.
The regenerative effect of bone grafts appears to depend on their chemical composition and structural properties, such as particle size, morphology, porosity, and solubility. Bovine bone, in particular, exhibits morphological similarities to human trabecular bone, contributing to its high biocompatibility and osteoconductivity. This resemblance is attributed to the presence of carbon in its hydroxyapatite structure, which closely matches that of human bone. Additionally, bovine bone resorbs at a slow but consistent rate, allowing it to remain at the graft site for extended periods. In contrast, synthetic grafts such as β-TCP exhibit higher solubility, which enhances osteoconductivity and bioresorbability (24).
Studies incorporating histological and histomorphometric evaluations support these findings, indicating that both bovine bone and synthetic biomaterials promote new bone formation (9-12, 15, 16, 18-22. In general, these materials exhibit osteoconductive properties and biocompatibility, as evidenced by the absence of inflammatory reactions, necrosis, or cellular rejection, along with clear signs of neovascularization (1, 9, 13, 14, 18-20, 22, 23).
The combination of hydroxyapatite and tricalcium phosphate (HA-TCP) has been reported to degrade faster than Bio-Oss, which may explain its higher percentage of new bone formation in certain studies. However, the slower resorption rate of Bio-Oss could be advantageous in cases requiring prolonged structural support (14). These findings align with those of Saleh et al., who reported histomorphometric evidence of well-organized Haversian canals colonized by cells and capillaries, along with active osteoid matrix deposition and mineralization on the surface of bovine bone grafts. The absence of inflammatory cell infiltration or foreign body reactions was also noted, further supporting the efficacy of bovine bone and calcium phosphate (CaP)-based ceramics, such as biphasic calcium phosphate (BCP) and β-TCP, in bone regeneration. Moreover, some studies have suggested that the combination of these biomaterials with other grafts or biologics may improve outcomes.
In animal models, Bio-Oss has been shown to exhibit clear osteoconductivity when surrounded by newly deposited bone, facilitating the optimal healing of the elevated sinus floor space. (25) Similarly, the use of a biphasic calcium phosphate graft (60% HA/40% β-TCP) has demonstrated excellent osteoconductivity across all observation periods (26).
Findings from multiple studies suggest that no statistically significant differences exist in new bone formation rates when using different biomaterials. However, the combination of materials represents a viable alternative to autologous bone grafts, achieving high levels of new bone formation within conventional healing periods (27).
Clinical Implications
The findings of this review suggest that both Bio-Oss and HA-TCP may provide beneficial outcomes in maxillary sinus lift procedures; however, the certainty of the evidence remains limited. The choice of biomaterial should therefore be tailored to patient-specific requirements and treatment objectives rather than assuming equivalence in performance:
- Bio-Oss may be preferable when long-term structural stability is prioritized, for example in patients who will receive implants expected to remain functional over extended periods.
- β-TCP, with its comparatively faster resorption, may be more suitable in cases where earlier bone turnover and integration are desirable.
- Cost considerations also play a role, as β-TCP is typically more affordable and may represent a viable option in specific clinical scenarios, although this should not be interpreted as conclusive evidence of cost-effectiveness.
No major adverse effects related to either biomaterial were reported in the included studies, though underreporting cannot be excluded.
Limitations and Future Recommendations
Interpretation of the present findings must take into account several limitations:
- Follow-up periods in most studies were short to medium term, limiting the assessment of long-term outcomes.
- Considerable methodological heterogeneity, including differences in study design, histological techniques, and outcome definitions, constrains comparability.
- The uneven number of studies per biomaterial further restricts balanced conclusions.
Future research should focus on well-designed randomized clinical trials with standardized histological and clinical protocols, longer follow-up periods, and direct head-to-head comparisons, including different HA/TCP ratios, to better determine their relative clinical performance.
Conclusions
Within the limitations of the available evidence, Bio-Oss and HA-TCP appear to be promising options for maxillary sinus bone regeneration, though differences exist in resorption profiles and new bone formation dynamics. Nonetheless, due to the heterogeneity of the studies, the unequal distribution of data between groups, and the overall risk of bias, the certainty of these conclusions remains low. Consequently, material selection should be individualized, and robust future studies are warranted to provide more definitive evidence and clinical guidance.
Conflict of interest: The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS STATEMENT: Conceptualization, Methodology, Software, Supervision, Data curation: J.J.U.V.; Validation: J.J.U.V. and M.S.V.; Investigation: J.J.U.V. and A.M.C.G.; Analysis, Display, Writing-original draft preparation, Drafting-revision and editing: J.J.U.V., A.M.C.G. and M.S.V.; Research: A.M.C.G. and M.S.V.
Funding: This research was developed by own resources from researchers.
REFERENCES
1. Carmagnola D., Pispero A., Pellegrini G., Sutera S., Henin D., Lodi G., et al. Maxillary sinus lift augmentation: A randomized clinical trial with histological data comparing deproteinized bovine bone grafting vs graftless procedure with a 5-12-year follow-up. Clin Implant Dent Relat Res. 2024; 26 (5): 972-85.
2. Kungvarnchaikul I., Subbalekha K., Sindhavajiva P.R., Suwanwela J. Deproteinized bovine bone and freeze-dried bone allograft in sinus floor augmentation: A randomized controlled trial. Clin Implant Dent Relat Res. 2023; 25 (2): 343-51.
3. Li X., Lin S-c, Duan S-y. The impact of deproteinized bovine bone particle size on histological outcomes in sinus floor elevation: a systematic review and meta-analysis. Int J Implant Dent. 2023; 9 (1): 35.
4. Krennmair S., Postl L., Schwarze U.Y., Malek M., Stimmelmayr M., Krennmair G. Clinical, radiographic, and histological/histomorphometric analysis of maxillary sinus grafting with deproteinized porcine or bovine bone mineral: A randomized clinical trial. Clin Oral Implants Res. 2023; 34 (11): 1230-47.
5. Starch-Jensen T., Schou S., Terheyden H., Bruun N.H., Aludden H. Bone regeneration after maxillary sinus floor augmentation with different ratios of autogenous bone and deproteinized bovine bone mineral an in vivo experimental study. Clinical Oral Implants Research. 2023; 34 (12):1406-16.
6. age M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; 372: n71.
7. Lyu X.Z., Sun F., Zhan S.Y. [Risk related to bias assessment: (4) Revised Cochrane Risk of Bias Tool for cluster-randomized control trials (RoB2.0)]. Zhonghua Liu Xing Bing Xue Za Zhi. 2018; 39 (2): 240-4.
8. Higgins J., Morgan R., Rooney A., Taylor K., Thayer K., Silva R., et al. A tool to assess risk of bias in non-randomized follow-up studies of exposure effects (ROBINS-E). Environ Int. 2024; 186: 7.
9. D'Alessandro D., Perale G., Milazzo M., Moscato S., Stefanini C., Pertici G., et al. Bovine bone matrix/poly(L-lactic-<i>co</i>-ε-caprolactone)/gelatin hybrid scaffold (SmartBone®) for maxillary sinus augmentation: A histologic study on bone regeneration. Int J Pharm. 2017; 523 (2): 534-44.
10. Kurkcu M., Benlidayi M.E., Cam B., Sertdemir Y. Anorganic bovine-derived hydroxyapatite vs b-tricalcium phosphate in sinus augmentation: A comparative histomorphometric study. J Oral Implantol. 2012; 38: 519-26.
11. Lee D.Z., Chen S.T., Darby I.B. Maxillary sinus floor elevation and grafting with deproteinized bovine bone mineral: A clinical and histomorphometric study. Clin Oral Implants Res. 2012; 23 (8): 918-24.
12. Martiniano C., Valadas L., do Carmo J., Alves A., Lotif M., Sotto-Maior B., et al. Research Article A Comparative Histomorphometric Analysis of Two Biomaterials for Maxillary Sinus Augmentation: A Randomized Clinical, Crossover, and Split-Mouth Study. Evid Based Complement Alternat Med. 2022; 2022.
13. Mummolo S., Nota A., Marchetti E., Capuano S., Tecco S., Marzo G., et al. Histologic and histomorphometric analysis of maxillary sinus augmentation with different biomaterials. A pilot split-mouth human study. Oral Implantol. 2018; 11 (4): 249-56.
14. Oh J-S, Seo Y-S, Lee G-J, You J-S, Kim S-G. A comparative study with biphasic calcium phosphate and deproteinized bovine bone in maxillary sinus augmentation: A prospective randomized controlled clinical trial. Int J Oral Maxillofac Implants. 2019; 34 (1): 233-42.
15. Pasquali P.J., Teixeira M.L., Oliveira T.A.D., De Macedo L.G.S., Aloise A.C., Pelegrine A.A. Maxillary Sinus Augmentation Combining Bio-Oss with the Bone Marrow Aspirate Concentrate: A Histomorphometric Study in Humans. Int J Biomater. 2015; 2015.
16. Pereira R., de Carvalho M., Hochuli-Vieira E., Statkievicz C., Santos D., Neto R., et al. Histomorphometric and Micro-CT Evaluation of Cerabone and Bio-Oss in Maxillary Sinus Lifting: A Randomized Clinical Trial. Medicina (Kaunas). 2024; 60 (11).
17. Pignaton T., Spin-Neto R., Ferreira C., Martinelli C., de Oliveira G., Marcantonio E., et al. Remodelling of sinus bone grafts according to the distance from the native bone: A histomorphometric analysis. Clin Oral Implants Res. 2020; 31 (10): 959-67.
18. Testori T., Wallace S., Trisi P., Capelli M., Zuffetti F., Del Fabbro M., et al. Effect of Xenograft (ABBM) Particle Size on Vital Bone Formation Following Maxillary Sinus Augmentation: A Multicenter, Randomized, Controlled, Clinical Histomorphometric Trial. Int J Periodontics Restorative Dent. 2013; 33 (4): 467-76.
19. Vincent-Bugnas S., Charbit Y., Charbit M., Dard M., Pippenger B. Maxillary sinus floor elevation surgery with BioOss mixed with enamel matrix derivative: A human randomized controlled clinical and histologic study. J Oral Implantol. 2020; 46 (5): 507-13.
20. Zhang Y., Tangl S., Huber C.D., Lin Y., Qiu L., Rausch-Fan X. Effects of Choukroun's platelet-rich fibrin on bone regeneration in combination with deproteinized bovine bone mineral in maxillary sinus augmentation: A histological and histomorphometric study. J Craniomaxillofac Surg. 2012; 40 (4): 321-8.
21. Zhou W., Wang F., Magic M., Zhuang M., Sun J., Wu Y. The effect of anatomy on osteogenesis after maxillary sinus floor augmentation: a radiographic and histological analysis. Clin Oral Investig. 2021; 25 (9): 5197-204.
22. Calasans-Maia M., Mourao C., Alves A., Sartoretto S., de Uzeda M., Granjeiro J., et al. Maxillary Sinus Augmentation with a New Xenograft: A Randomized Controlled Clinical Trial. Clin Implant Dent Relat Res. 2015; 17: E586-E93.
23. Ohayon L., Taschieri S., Corbella S., Del Fabbro M., Ohayon L., Taschieri S., et al. Maxillary Sinus Floor Augmentation Using Biphasic Calcium Phosphate and a Hydrogel Polyethylene Glycol Covering Membrane: An Histological and Histomorphometric Evaluation. Implant Dent. 2016; 25 (5): 599-605.
24. Sato N., Handa K., Venkataiah V.S., Hasegawa T., Njuguna M.M., Yahata Y., et al. Comparison of the vertical bone defect healing abilities of carbonate apatite, β-tricalcium phosphate, hydroxyapatite and bovine-derived heterogeneous bone. Dent Mater J. 2020; 39 (2): 309-18.
25. Scala A., Lang N.P., Velez J.U., Favero R., Bengazi F., Botticelli D. Effects of a collagen membrane positioned between augmentation material and the sinus mucosa in the elevation of the maxillary sinus floor. An experimental study in sheep. Clin Oral Implants Res. 2016; 27 (11): 1454-61.
26. Favero V., Lang N.P., Canullo L., Urbizo Velez J., Bengazi F., Botticelli D. Sinus floor elevation outcomes following perforation of the Schneiderian membrane. An experimental study in sheep. Clin Oral Implants Res. 2016; 27 (2): 233-40.
27. Trimmel B., Gede N., Hegyi P., Szakács Z., Mezey G.A., Varga E., et al. Relative performance of various biomaterials used for maxillary sinus augmentation: A Bayesian network meta-analysis. Clin Oral Implants Res. 2021; 32 (2): 135-53.
 Odovtos -Int J Dent Sc endoses to CC-BY-NC-SA 4.0.
Odovtos -Int J Dent Sc endoses to CC-BY-NC-SA 4.0.