BASIC RESEARCH:
Effect of Relationship Between β-Catenin and Extracellular Matrix on Progression of Oral Epithelial Dysplasia to Oral Squamous Cell Carcinoma
Efecto de la relación entre la Matriz Extracelular y β-Catenina en la progresión de la Displasia Oral Epitelial a Carcinoma Oral de Células Escamosas
Seham Ahmed Abdel Ghani¹ https://orcid.org/0000-0001-8100-3833
Amany A. Rabea2 https://orcid.org/0000-0001-7139-2572
¹Assistant Professor of Oral Pathology, Faculty of Dentistry, Ain Shams University, Cairo, Egypt.
2Professor of Oral Biology, Faculty of Oral and Dental Medicine, Future University in Egypt, Cairo, Egypt.
Correspondence to: Amany A. Rabea - amany.ahmed@fue.edu.eg
Received: 30-I-2025 Accepted: 21-V-2025
ABSTRACT: This study aimed to explore roles of β-catenin, collagen&elastic fibers in progression of Oral Epithelial Dysplasia (OED) to oral squamous cell carcinoma (OSCC). Sixty paraffin wax blocks of oral mucosa were divided into: control (Group I), mild OED (Group II), severe OED (Group III), well differentiated SCC (Group IV), moderately differentiated SCC (Group V), poorly differentiated SCC (Group VI). The sections underwent staining using hematoxylin and eosin, histochemical stains and β-catenin immunostaining. Group I revealed normal gingival tissue. Group II showed some basal and prickle cells with pleomorphic nuclei. Group III illustrated poorly demarcated cell membrane. Group IV showed keratinized epithelial islands. Group V was devoid of keratin pearls. Group VI showed dispersed epithelial cells. β-catenin-immunoreactivity was; strong membranous in Group I, moderate membranous, cytoplasmic and nuclear in Groups II&III, moderate nuclear and cytoplasmic in Group IV but strong in Groups V&VI. Collagen fibers were closely packed in Groups I&II, loosely packed in Groups III&IV, dispersed in Groups V&VI. Elastic fibers in Group I were apparently few & thin, abundant in Group II, numerous in Group III, short in Groups IV&V and thick in Group VI. β-catenin and elastic fibers were upregulated meanwhile collagen formation was downregulated towards progression from mild OED to poorly differentiated SCC.
KEYWORDS: β-Catenin; Elastic fibers; Collagen; Oral epithelial dysplasia; Oral squamous cell carcinoma.
RESUMEN: El objetivo del presente estudio es explorar el rol de β-catenina, las fibras elásticas y de colágeno en la progresión de la Displasia Oral Epitelial (DOE) hacia el Carcinoma oral de células escamosas (COCE). Se utilizaron seis muestras de mucosa oral, montados en bloques de parafina, los cuales se dividieron en: (Grupo I) control, (Grupo II) medio DOE, (Grupo III) DOE severo, (Grupo IV) COCE bien diferenciado, (Grupo V) COCE moderadamente diferenciado, (Grupo VI) COCE poco diferenciado. L A las muestras se les realizó análisis histológico con hematoxilina-eosina, y análisis de inmunohistoquímica con marcadores para β-catenina. El Grupo I mostró tejido gingival normal. El Grupo II presentó algunas células basales y del estrato espinoso con núcleos pleomórficos. El Grupo III evidenció membranas celulares mal delimitadas. El Grupo IV mostró islas de epitelio queratinizadas. El Grupo V carecía de perlas de queratina. El Grupo VI presentó células epiteliales dispersas. La inmunorreactividad para β-catenina se observó intensa a nivel de la membrana en el Grupo I; localización moderada a nivel de membrana, citoplasma y núcleo en los Grupos II y III; en el Grupo IV localización moderada a nivel de núcleo y citoplasma, pero intensa en los Grupos V y VI. Las fibras colágenas se observaron densamente compactadas en los Grupos I y II, laxamente organizadas en los Grupos III y IV, y dispersas en los Grupos V y VI. Las fibras elásticas fueron escasas y delgadas en el Grupo I, abundantes en el Grupo II, numerosas en el Grupo III, cortas en los Grupos IV y V, y gruesas en el Grupo VI. La expresión de β-catenina y de las fibras elásticas se encontró aumentada, mientras que la formación de colágeno se observó disminuida a medida que progresaba la lesión desde una displasia epitelial oral (DEO) leve hasta un carcinoma escamoso poco diferenciado.
PALABRAS CLAVE: β-Catenina; Fibras elásticas; Colageno; Displasia epitelia 0ral; Carcinoma oral de células escamosas.
Copyright (c) 2025 Seham Ahmed Abdel Ghani, Amany A. Rabea.

Odovtos -Int J Dent Sc endoses to CC-BY-NC-SA 4.0.
Introduction
Oral cancer ranks as the sixth most prevalent malignant tumor globally, with oral squamous cell carcinoma (OSCC) making up approximately 90% of all oral malignancies (1, 2).
OSCC is preceded by oral potentially malignant disorders (OPMDs) which involve morphological changes in oral epithelium and diagnosed as oral epithelial dysplasia (OED). According to the World Health Organization, two methods were used for classification of OED: 1st is the grading system, which includes: mild, moderate and severe OED (3-5), 2nd categorization involve: low-grade and high-grade OED and this is the most recent used method (6).
The Wnt signaling includes two pathways; canonical and noncanonical, they are involved in different biological functions, as cell differentiation, proliferation and migration (7, 8). The canonical Wnt pathway (Wnt/β-catenin) is the most commonly studied pathway, because it is mutated in most malignant neoplasms, specially, in oral premalignant disorders and oral malignances (9-18).
The Wnt/β-catenin signaling pathway is upregulated when extracellular Wnt ligands are attached to their membrane receptors through autocrine or paracrine signaling. This results in the destabilization and nuclear translocation of β-catenin, which in turn upregulates genes expression participated in cell proliferation, differentiation, viability, and migration (19, 20). Moreover, β-catenin is necessary for stromal fibroblasts to perform its remodeling effect on extracellular matrix (ECM) and to obey to the malignant cells stimulatory signals (21).
The ECM is the most common component of the tumor microenvironment which helps tumor progression through biochemical modulation of tumor cells (22-24). Collagen is main component of ECM which is secreted by numerous stromal cells especially cancer associated fibroblasts (25-27). Collagen plays an essential role in stabilizing the physical integrity of the tissues, as morphogenesis, adhesion, migration, differentiation, and tissue repair (28-31). Moreover, collagen can stimulate tumor cell growth and metastasis (32).
Elastic fibers are the second important element of ECM which consist of different proteins as elaunin, elastin, and oxytalan (33). They are important for normal organ elasticity (34). Although, there is association between elastic fibers and cancer cells; its effect on cancer progression is not clear (35).
Although, OED have high tendency to progress into OSCC, however the exact mechanism that explains OED transformation to OSCC is not well understood. On the other hand, these lesions consist of two components i.e. epithelial cells and ECM, which interact and affect each other and change the biological behavior of oral tumor (36). In this study, we aimed to explore the role of β-catenin, collagen and elastic fibers in the progression of OED to OSCC.
Materials and methods
Study design and specimen preparation
This study was conducted in compliance with the ethical guidelines established by the Research Ethics Committee at Ain Shams University (FDASU-Rec EM072323).
The inclusion and exclusion criteria are established depend on factors that ensure the sample's relevance and quality for the study. In this study, we selected well-preserved tissue in paraffin blocks exhibiting clear cellular morphology, sourced from patients with the target condition, ensuring an adequate sample size and available histopathological data. Furthermore, appropriate storage conditions were maintained to prevent tissue degradation. Conversely, tissue samples that were degraded or of poor quality, with unclear staining or loss of detail, incomplete or insufficient data, non-representative samples that did not match the condition being studied, or samples that were contaminated or improperly processed, were excluded from our study. These criteria are essential for ensuring that the data obtained from the tissue samples is reliable, accurate, and consistent with the research objectives.
The study involved 60 paraffin wax blocks of human oral mucosa, divided into six groups each group consisted of 10 cases: Group I (negative control) revealed normal epithelial gingiva, Group II (mild OED), Group III (severe OED), Group IV well-differentiated SCC (WDSCC), Group V moderately-differentiated SCC (MDSCC), and Group VI poorly-differentiated SCC (PDSCC) which served as the positive control groups.
Paraffin embedded tissue blocks were from the archives of the Oral Pathology Department at the Faculty of Dentistry, Ain Shams University. The blocks were sectioned at 4 um thickness, four sections from each block were obtained, placed on the slides and incubated at 47°C for 1 h, then deparaffinized in xylene and hydrated in descending grades of alcohol, finally rinsed with water (37). Four sets of sections were subjected to staining with hematoxylin and eosin (H&E), Masson Goldner’s trichrome. and Orcein special stains as well as β-catenin immunolocalization. The specimens were analyzed using a light microscope (Olympus® BX 60, Tokyo, Japan) and slides were photographed at 400× magnifications.
H&E staining
The sections were stained by H&E (Sigma®, St. Louis, MO, USA) to confirm the diagnosis of the lesions (38).
Immunohistochemical staining
The sections were placed on positively charged slides and were microwaved for 20 minutes in 0.01mol/L citrate buffer (pH=6) to retrieve the antigen. Endogenous peroxidase activity was blocked using 3% hydrogen peroxide, and non-specific binding was prevented by incubating the sections in goat serum for 30 minutes. Then, treated with rabbit polyclonal primary antibody (1:200 diluted) specific to β-catenin (Thermo Scientific, Fremont, CA). After that, The sections were incubated with a biotinylated secondary antibody (Serotec®, Bio-Rad, USA), followed by washing with phosphate buffer and incubation with streptavidin (Serotec®, Bio-Rad, USA). To visualize the results, the sections were treated with diaminobenzidine (Serotec®, Bio-Rad, USA), producing a brown color (39, 40).
Masson Goldner's trichrome special staining
The sections were stained using Masson Goldner’s trichrome special stain (Sigma®, St. Louis, MO, USA) as follows, Bouin's solution was applied over night at room temperature, then cooling of the sections was performed for 10 minutes at room temperature, then washing for 10 seconds in distilled water. Addition of Hematoxylin, Weigert A and Ferri reagent, Weigert B (5 drops) for 15-20 minutes, and rinsing under tap water for 3 minutes was carried out. Ten drops of Biebrich Scarlet-Acid Fuchsin reagent was applied for 20 minutes to remove the excess dye. Followed by distilled water rinsing and ten drops of PTA-PMA reagents for 10 minutes. Then 10 drops of Fast Green F.C.F reagent was used for 5-7 minutes and rinsed with distilled water. Finally, slides were treated with 1% acetic acid solution for 1-2 seconds, dehydrated using Histanol 100 for 2 minutes, and cleared in xylene 2 times, for 2 minutes (41, 42). This method was applied to identify newly formed collagen, which appears green.
Orcein special staining
The sections were incubated in oxidizing solution for 10 minutes, then rinsed with running water. 3-5 drops of oxalic acid solution (2%) were applied on tissue sections for 10 minutes. Then sections were rinsed for 1 minute in running tap water. After that 3-5 drops of orcein solution were applied on tissue sections that incubated afterwards for 2 h. The slides were washed in alcohol 70%, then 3-5 drops of differentiating solution were applied for 3-5 seconds. Dehydration 3 times using absolute alcohol was done and finally the slides were cleared (43, 44). This staining technique was used to detect elastic fibers in the color red-brown.
Histomorphometric assessment
Histomorphometric analysis was done on both immunolabeled and specially stained sections using Image J software (version 1.8.0; National Institutes of Health, Bethesda, MD, USA). The specimens were assessed to measure the area percentage of β-catenin immunoreactivity, newly formed collagen, and elastic fibers. Four distinct fields of each specimen were analyzed at a magnification of x400.
Statistical analysis
The values are expressed as mean±standard deviation (± SD). Normality of the data was assessed using the Kolmogorov-Smirnov test. The Kolmogorov-Smirnov test results showed that the recorded values followed a normal distribution. Therefore, a one-way analysis of variance (ANOVA) was conducted to compare the groups, with Tukey’s post hoc test applied for pairwise comparisons. The significance level was set at p≤0.05. Statistical analysis was conducted using SPSS 18.0 (Statistical Package for the Social Sciences, SPSS, Inc., Chicago, IL, USA) for Windows operating system.
Results
Histological results
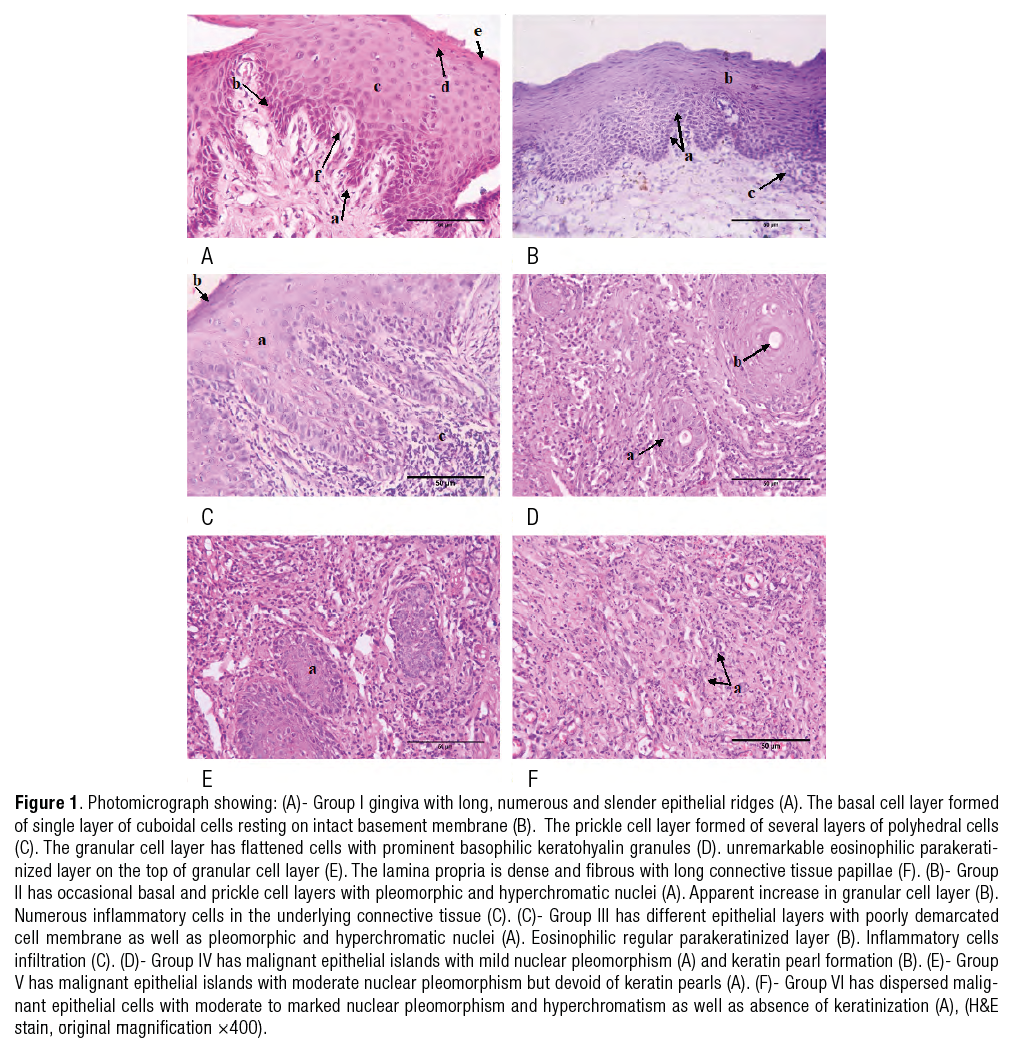
Group I revealed epithelial gingiva covered by parakeratinized stratified squamous epithelium with long, numerous and slender epithelial ridges. The basal cell layer was formed of single layer of cuboidal shape cells with large round basophilic nucleus resting on intact basement membrane. The prickle cell layer was formed of several layers of polyhedral cells. The granular cell layer exhibited flattened cells with prominent basophilic keratohyalin granules in their cytoplasm. unremarkable eosinophilic parakeratinized layer was detected on the top of granular cell layer. The underlying connective tissue appeared dense and fibrous with long connective tissue papillae (Figure 1.A).
Group II showed occasional basal and prickle cell layers with pleomorphic and hyperchromatic nuclei. Apparent increase in granular cell layer was detected. Numerous inflammatory cells in the underlying connective tissue were observed (Figure 1.B).
Group III illustrated different epithelial layers with poorly demarcated cell membrane as well as pleomorphic and hyperchromatic nuclei. However eosinophilic regular parakeratinized layer was noticed. Inflammatory cells infiltrate the underlying connective tissue (Figure 1.C).
Group IV illustrated malignant epithelial islands with mild nuclear pleomorphism showing keratin pearl formation (Figure 1.D).
Group V explored malignant epithelial islands with moderate nuclear pleomorphism but devoid of keratin pearls (Figure 1.E).
Group VI showed dispersed malignant epithelial cells with moderate to marked nuclear pleomorphism and hyperchromatism as well as absence of keratinization (Figure 1.F).
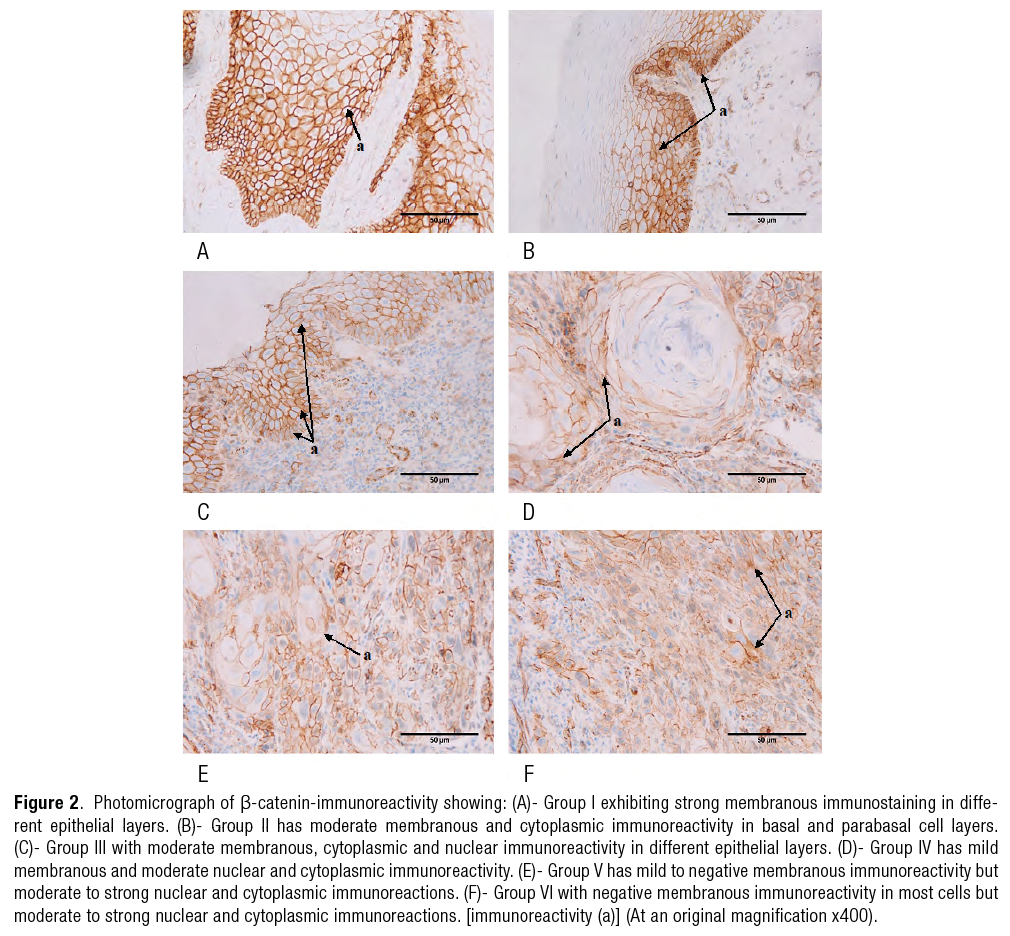
Immunohistochemical analysis results (FigURE 2.a-2.f).
|
Group |
β-catenin-immunoreactivity |
|
Group I |
Showed strong membranous immunoreactivity in different epithelial layers. |
|
Group II |
Showed moderate membranous and cytoplasmic immunoreactivity in basal and parabasal cell layers. |
|
Group III |
Illustrated moderate membranous and cytoplasmic immunoreactivity in different epithelial layers. |
|
Group IV |
Explored mild membranous, moderate nuclear and cytoplasmic immunoreactivity. |
|
Group V |
Explored mild to negative membranous immunoreactivity but moderate to strong nuclear and cytoplasmic immunoreactions. |
|
Group VI |
Showed negative membranous immunoreactivity in most cells but moderate to strong nuclear and cytoplasmic immunoreactions. |
Histochemical results
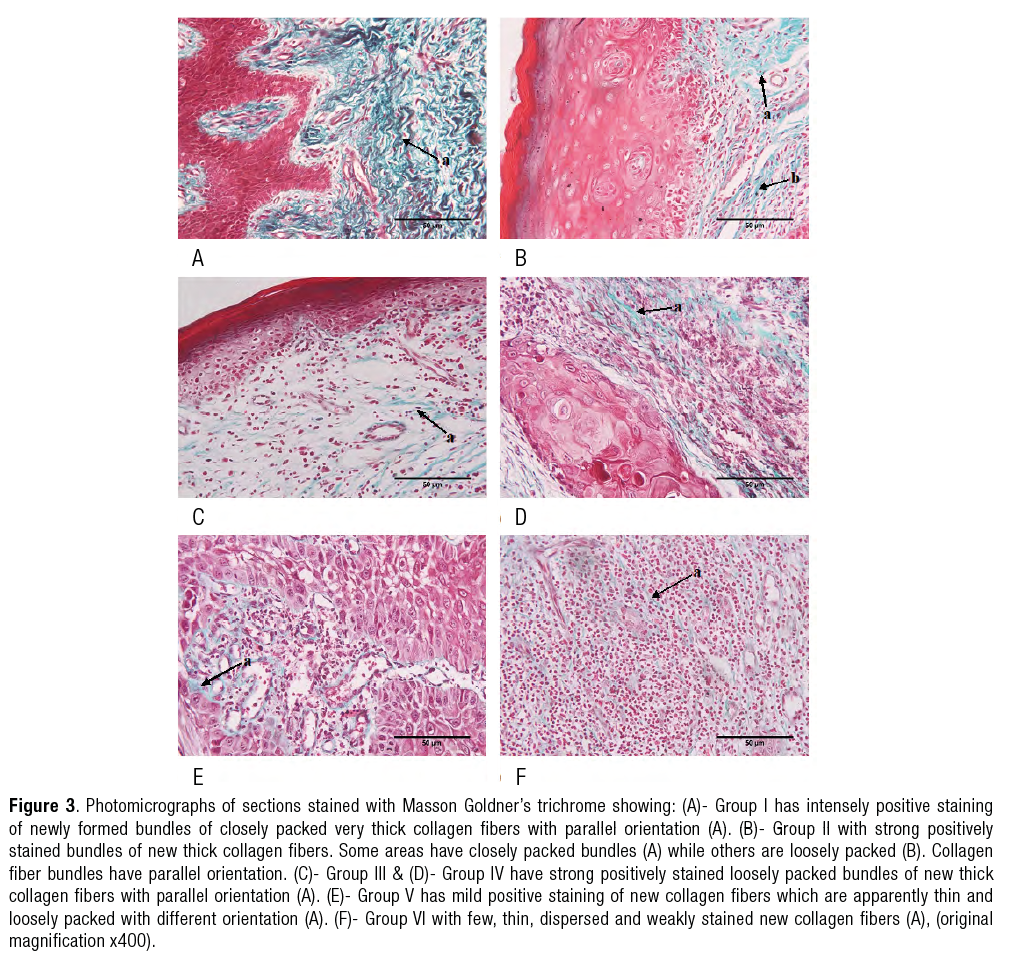
Masson Goldner’s trichrome results
(Figure 3.a-3.f)
|
Group |
Masson Goldner’s trichrome stain |
|
Group I |
Revealed intensely positive staining of newly formed bundles of closely packed very thick collagen fibers with parallel orientation. |
|
Group II |
Showed strong positively stained bundles of new thick collagen fibers. Some areas showed closely packed bundles while others were loosely packed. Collagen fiber bundles had parallel orientation. |
|
Group III & Group IV |
Explored strong positively stained loosely packed bundles of new thick collagen fibers with parallel orientation. |
|
Group V |
New collagen fibers showed mild positive staining, they were apparently thin and loosely packed with different orientation. |
|
Group VI |
Showed few, thin, dispersed and weakly stained new collagen fibers. |
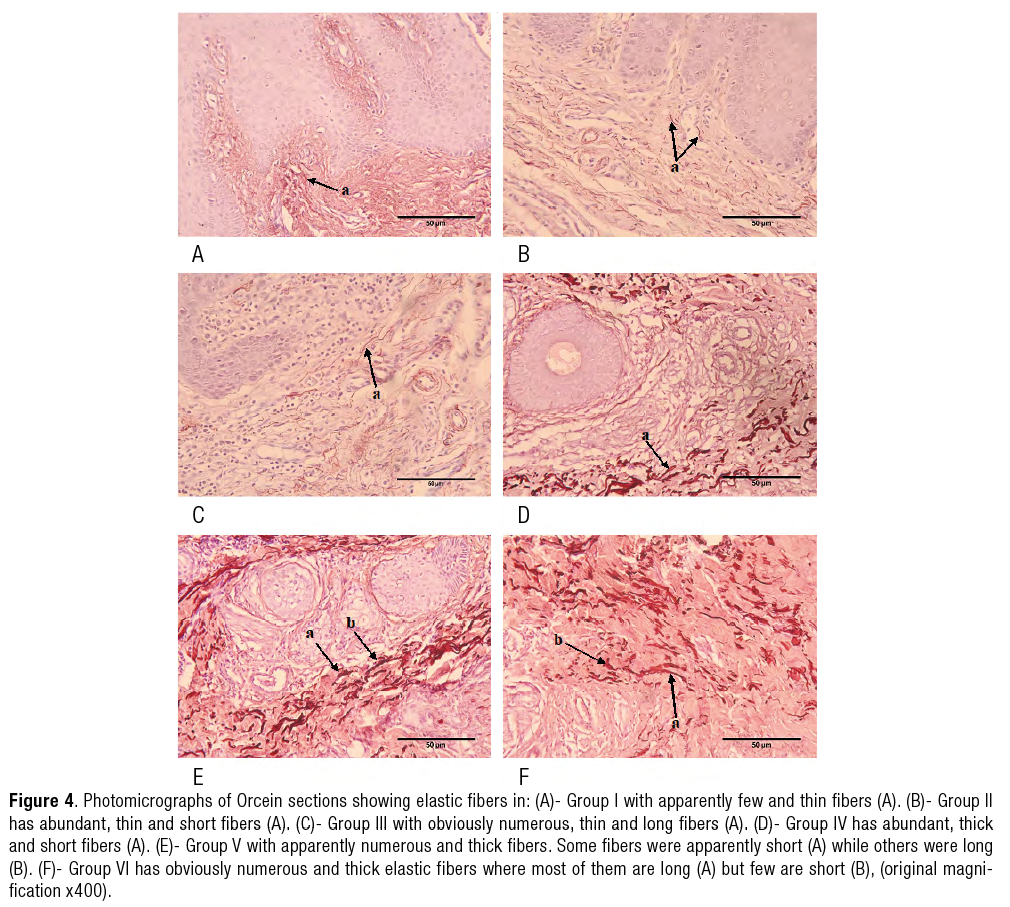
Orcein stain results (Figure 4.a-4.f)
|
Group |
Orcein stain |
|
Group I |
Elastic fibers were apparently few and thin. |
|
Group II |
They were abundant, thin and short. |
|
Group III |
Illustrated obviously numerous, thin and long elastic fibers. |
|
Group IV |
Explored abundant, thick and short elastic fibers. |
|
Group V |
Elastic fibers were apparently numerous and thick. Some fibers were apparently short while others were long. |
|
Group VI |
Showed obviously numerous and thick elastic fibers where most of them were long but few were short. |
Statistical results
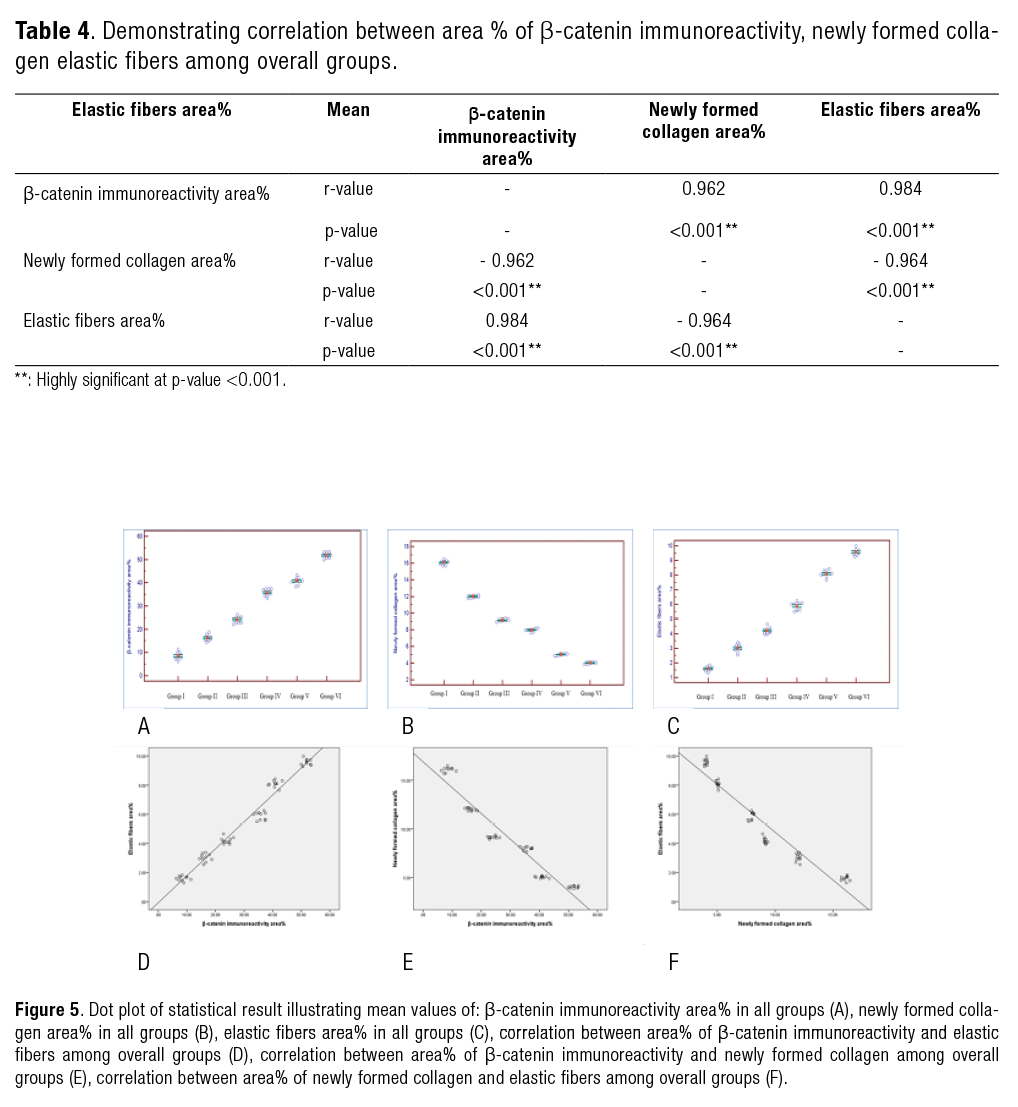
There was statistically significant difference between studied groups in the measured parameters. Both area percentage of β-catenin immunoreactivity (Table 1 & Figure 5.A) and elastic fibers (Table 3 & Figure 5.C) showed that Group VI has the highest mean followed by Groups V, IV, III, II and I respectively. While, area percentage of newly formed collagen explored the highest mean in Group I followed by Groups II, III, IV, V and VI respectively (Table 2 & Figure 5.B).
There was a statistically significant positive correlation between β-catenin immunoreactivity area percentage and elastic fibers area percentage, while statistically significant negative correlation between β-catenin immunoreactivity area percentage and newly formed collagen area percentage, as well as negative correlation between newly formed collagen area percentage and elastic fibers area percentage (Table 4 & Figure. 5.D, 5.E, 5.F).
Discussion
A significant proportion of oral cancer is believed to develop from precancerous or potentially cancerous lesions or conditions, collectively known as OPMDs (3).
It is assumed that mild OED may advance to severe OED and ultimately lead to carcinoma. This tissue transformation is thought to result from abnormal genetic modulation and signaling, as well as altered protein expression. It has been observed that the tissue formed by altered cells is distinct from that produced by normal cells (45).
In this study, immunohistochemical stains showed that; comparing to normal oral mucosa, membranous β-catenin expression is significantly reduced accompanied by a change in the localization of β-catenin expression in the cytoplasm and/or nuclei with increasing staining intensity from mild and severe OED cases, towards different grades of OSCC as PDSCC expresses the greatest nuclear expression. This could be explained by the fact that the Wnt/β-catenin signaling pathway is triggered when extracellular Wnt ligands bind to a membrane receptor in an autocrine or paracrine fushion. Upon activation, this pathway promotes the stabilization and nuclear translocation of β-catenin, which plays a key role in regulating the expression of genes involved in cell proliferation, survival, differentiation, and migration (20). However, the Wnt/β-catenin signaling pathway is controlled by Wnt antagonists, as a result, a balanced interplay between Wnt ligands and their inhibitors controls cell proliferation in normal tissues (46). But in different cancers, decreased expressions of these antagonists lead to the accumulation of β-catenin in the cytoplasm, followed by its translocation to the nucleus, where it activates target genes such as c-myc and cyclin D1 triggering cell proliferation and upregulation of tumor progression (47, 48).
The Masson Goldner’s trichrome results in the current study are in line with those of previous studies which explained the decrease in amount, and density as well as variations in alignment of mature collagen fibers as lesions progressed from mild OED ending by PDSCC is based upon the abnormal collagen production and defective maturation, which may promote the neoplastic growth (49). Moreover, cancer cells continuously secrete collagen-destroying enzymes (collagenases, proteinases), resulting in the degradation of the surrounding collagen. This leads to the dissolution or immaturity of collagen fibers, which facilitates tumor growth and metastasis (37).
In the present research, the orcein stain results could be attributed to the fact that the response of the extracellular matrix to tumor cell and lymphocyte-induced damage may result in increased production of elastic fibers over time. Moreover, the cancer cells can interact specifically with elastin through elastin binding proteins. This chemotactic effect of elastin peptides on cancer cells has a positive correlation with tumor progression (50, 51).
In addition, our statistical results revealed positive correlation between area percentage of β-catenin immunoreactivity and elastic fibers, while negative correlation was recorded between area percentage of both β-catenin immunoreactivity and newly formed collagen, as well as area percentage of newly formed collagen and elastic fibers. This could be attributed to the aid of type I collagen, which binds to integrins, triggering the focal adhesion kinase (FAK) and integrins-p38 mitogen-activated protein kinase (MAPK) pathways to stimulate matrix metalloproteinase activity (collagen-destroying enzymes) and phosphorylates FAK which then induces dissociation of β-catenin from E-cadherin ,allowing it to enter the nucleus. Moreover, β-catenin stimulates fibroblasts and possibly other cells to produce elastic tissue which affects tumor progression (52-55).
Conclusions
The basis of tissue alteration in mild OED and its progression to severe OED and then to carcinoma is abnormal signaling, as well as altered protein expression. β-catenin immunoreactivity and elastic fibers expression are upregulated meanwhile newly formed collagen is downregulated towards the progression from mild OED up to PDSCC.
Clinical relevance
The clinical significance of these findings in the progression from mild OED to PDSCC lies in the potential use of β-catenin immunoreactivity and elastic fiber expression as valuable biomarkers. These markers could aid in the early diagnosis and prediction of OED progression to carcinoma, offering crucial insights for clinical management and intervention strategies.
Diagnosis: The elevated expression of β-catenin and alterations in elastic fiber expression could serve as crucial markers for detecting early-stage dysplastic changes in the oral epithelium. These biomarkers may aid in differentiating between mild and severe dysplasia, thereby improving the accuracy of identifying potentially malignant lesions in clinical practice.
Prognosis: The altered expression of β-catenin, often associated with disrupted cell signaling and tumor progression, along with the downregulation of collagen, may indicate an elevated risk of progression to carcinoma, particularly in severe OED. This could help clinicians identify patients at greater risk for malignant transformation, allowing for more proactive monitoring and intervention strategies.
Therapeutic Implications: Understanding the molecular changes involved in the transition from OED to PDSCC could reveal new therapeutic targets. Targeting the β-catenin signaling pathway or restoring normal collagen production might provide promising strategies for developing treatments aimed at preventing or slowing malignant progression.
The study has certain limitations, such as the lack of long-term follow-up to observe disease progression, the potential absence of demographic diversity within the sample, and the possibility of technical variability in methods or confounding factors that may affect the results. These limitations highlight the necessity for larger, more inclusive studies with prolonged follow-up periods to further validate and confirm the findings.
Funding
This research is self-financed.
Declaration of interest
No conflict of interest.
Consent to Participate
Not Applicable
AUTHOR CONTRIBUTION STATEMENT
The conception and design of the study, acquisition, analysis and interpretation of data: S.A.A.G. and A.A.R.
Drafting the article and revising it critically for important intellectual content: S.A.A.G. and A.A.R.
Final approval of the version to be submitted: S.A.A.G. and A.A.R.
References
1. Lala M., Chirovsky D., Cheng J.D., Mayawala K. Clinical outcomes with therapies for previously treated recurrent/metastatic head-and-neck squamous cell carcinoma (R/M HNSCC). a systematic literature review. Oral Oncol. 2018; 84: 108-120.
2. Siegel R.L., Miller K.D., Jemal A. Cancer statistics.CA Cancer J Clin. 2019; 69 (1): 7-34.
3. Warnakulasuriya S., Johnson N., van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med. 2007; 36: 575 580.
4. Warnakulasuriya S., Reibel J., Bouquot J., Dabelsteen E. Oral epithelial dysplasia classification systems: Predictive value, utility, weaknesses and scope for improvement. J. Oral. Pathol. Med. 2008; 37: 127-133.
5. Fleskens S., Slootweg PJ. Grading systems in head and neck dysplasia: Their prognostic value, weaknesses and utility. Head Neck Oncol. 2009; 1: 1-11.
6. El-Naggar A., Chan J., Takata T., Grandis J., Slootweg P.J. The fourth edition of the head and neck World Health Organization blue book Editors’ perspectives. Hum. Pathol. 2017; 66: 10-12.
7. Logan C., Nusse R. The wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Boil. 2004; 20: 781-810.
8. Nusse R. Wnt signaling. Cold Spring Harb. Perspect. Boil. 2012; 4: a011163.
9. Morin P., Sparks A., Korinek V., Barker N., Clevers H., Vogelstein B., Kinzler K. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science. 1997; 275: 1787-1790.
10. Balint K., Xiao M., Pinnix C., Soma A., Veres I., Juhasz I., Brown E., Capobianco A., Herlyn M., Liu Z. Activation of Notch1 signaling is required for beta-catenin-mediated human primary melanoma progression. J. Clin. Investig. 2005; 115: 3166-3176.
11. Khalil S., Tan G., Giri D., Zhou X., Howe L. Activation status of Wnt/ß-catenin signaling in normal and neoplastic breast tissues: Relationship to HER2/NEU expression in human and mouse. PLoS ONE. 2012; 7: e33421.
12. Santoro A., Pannone G., Papagerakis S., McGu H., Cafarelli B., Lepore S., De Maria S., Rubini C., Mattoni M., Staibano S. Beta-catenin and epithelial tumors: A study based on 374 oropharyngeal cancers. BioMed Res. Int. 2014; 2014: 1-13.
13. Zhan P., Zhang B., Xi G., Wu Y., Liu H., Liu Y., Xu W., Zhu Q., Cai F., Zhou Z. PRC1 contributes to tumorigenesis of lung adenocarcinoma in association with the Wnt/β - catenin signalingpathway. Mol. Cancer. 2017; 16: 108.
14. Sato K., Okazaki Y., Tonogi M., Tanaka Y., Yamane G. Expression of beta-catenin in rat oral epithelial dysplasia induced by 4-nitroquinoline 1-oxide. Oral Oncol. 2002; 38: 772-778.
15. Ravindran G., Devaraj H. Aberrant expression of beta-catenin and its association with DeltaNp63, Notch-1, and clinicopathological factors in oral squamous cell carcinoma. Clin. Oral. Investig. 2012; 16: 1275-1288.
16. Kaur J., Sawhney M., DattaGupta S., Shukla N., Srivastava A., Walfish P., Ralhan R. Clinical significance of altered expression of beta-catenin and E-cadherin in oral dysplasia and cancer: Potential link with ALCAM expression. PLoS ONE. 2013; 8: e67361.
17. Reyes M., Rojas-Alcayaga G., Maturana A., Aitken J., Rojas C., Ortega A. Increased nuclear beta-catenin expression in oral potentially malignant lesions: A marker of epithelial dysplasia. Med. Oral. Patol. Oral. Cir. Bucal. 2015; 20: 540.
18. Marimuthu M., Andiappan M., Wahab A., Muthusekhar M., Balakrishnan A., Shanmugam S.C. Wnt pathway gene expression and their clinical correlation in oral squamous cell carcinoma. Indian J. Dent. Res. 2018; 29: 291-297.
19. Willert K., Nusse R. Wnt Proteins. Cold Spring Harb. Perspect. Boil. 2012; 4: a007864.
20. Cruciat C., Niehrs C. Secreted and transmembrane Wnt inhibitors and activators. Cold Spring Harb. Perspect. Boil. 2012; 5: a015081.
21. Liu T., Zho L., Yang K., Iwasawa K., Kadekaro A., Takebe T., Andl T., Zhang Y. The β-catenin/YAP signaling axis is a key regulator of melanoma-associated fibroblasts. Signal Transduction and Targeted Therapy. 2019; 4: 63-78.
22. Lu P., Weaver V., Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012; 196: 395-406.
23. Chen P., Cescon M., Bonaldo P. Collagen VI in cancer and its biological mechanisms. Trends Mol Med. 2013; 19: 410-417.
24. Walker C., Mojares E., Del Río H. Role of extracellular matrix in development and cancer progression. Int J Mol Sci. 2018; 19: 3028.
25. Ricard-Blum, S. The collagen family. Cold Spring Harb Perspect Biol. 2011; 3:a004978.
26. Sun Q, Zhang B, Hu Q. The impact of cancer-associated fibroblasts on major hallmarks of pancreatic cancer. Theranostics. 2018; 8: 5072-5087.
27. Baglieri J., Brenner D., Kisseleva T. The role of fibrosis and liver associated fibroblasts in the pathogenesis of hepatocellular carcinoma. Int J Mol Sci. 2019; 20: 1723.
28. Myllyharju J., Kivirikko K. Collagens and collagen-related diseases.Ann Med. 2001; 33: 7-21.
29. Badylak S. The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Dev Biol. 2002; 13: 377-383.
30. Swinehart I., Badylak S. Extracellular matrix bioscaffolds in tissue remodeling and morphogenesis. Dev Dyn. 2016; 245: 351-360.
31. El Deeb M., Rabea A.A. Therapeutic Effect of Curcumin, Ginger and Tamarind on Oral and Paraoral tissues: Histological Overview. Future Dental Journal. 2023; 9: 1-9.
32. Xu S., Xu H., Wang W., Li S., Li H., Li T., Zhang W., Yu X., Liu L. The role of collagen in cancer: from bench to bedside. J Transl Med. 2019;17: 309-331.
33. Kielty C., Sheratt M., Shuttleworth C. Elastic fibres. J Cell Sci. 2002; 115: 2817-2828.
34. Uitto J. Biochemistry of the elastic fibers in normal connective tissues and its alterations in diseases. J Invest Dermatol. 1979; 72: 1-10.
35. Kardam P., Mehendiratta M., Rehani S., Kumra M., Sahay K., Jain K. Stromal fibers in oral squamous cell carcinoma: a possible new prognostic indicator? J Oral Maxillofac Pathol. 2016; 20: 405-412.
36. Clark A., Vignjevic D. Modes of cancer cell invasion and the role of the microenvironment. Curr Opin Cell Biol. 2015; 36: 13-22.
37. Khan S., Hashmi S., Vij H. Histopathological Evaluation of Connective Tissue Stroma in Oral Squamous Cell Carcinoma. A Histochemical study BrJ Med Health Res. 2020; 7 (06): 95-106. https://pubmed.ncbi.nlm.nih.gov/12082143/
38. Shredah M., El-Sakhawy M. Immunohistochemical expression of activated caspase-3 in the parotid salivary glands of rats after long administration of Myristica fragrans. International Journal of Advanced Research. 2014; 2 (12): 493-499.
39. Kim T., Lee J., Baek J., Lee J., Yang X., Taketo M., Jiang R., Cho E. Constitutive stabilization of β-catenin in the dental mesenchyme leads to excessive dentin and cementum formation. Biochem. Biophys. Res. Commun. 2011; 412: 549-555.
40. Rajabi P., Heydarpoor M., Maghsoudi A., Mohaghegh F., Mobarakeh M. The study for diagnostic value of β-catenin immunohistochemistry marker in distinction of aggressive and non aggressive basal cell carcinoma. Iran J. Pathol. 2019; 14 (1): 52-60.
41. Melis M., Carpino F., Di Tondo U. Techniques in pathological anatomy: autopsies, photon microscopy, histology, electron microscopy, cytology, cytogenetics: cytopathological diagnostics. 1989; (ed. 19). (Chapter 16) p 491.
42. Bancroft J., Gamble M. Theory and practice of Histological Techniques, Churchill Livingstone. New York. 2002; (P 63-84).
43. Deodhar K., Tapp E., Scheuer P. Orcein staining of Hepatitis B Antigen in paraffin sections of Liver Biopsies. Journal of Clinical Pathology. 1975; 28: 66-70.
44. Salaspuro M., Sipponen P. Demonstration of an intracellular copper-binding protein by Orcein staining in long-standing cholestatic liver diseases. Gut. 1976;17: 787-790.
45. Speight P.M. Update on oral epithelial dysplasia and progression to cancer. Head Neck Pathol. 2007; 1: 61-66.
46. Voronkov A., Krauss S. Wnt/beta-catenin signaling and small molecule inhibitors. Curr. Pharm. Des. 2013; 19: 634-664.
47. Komiya Y., Habas R. Wnt signal transduction pathways. Organogenesis. 2008; 4:68-75. doi: 10.4161/org.4.2.5851.
48. Xiao C., Wang L., Zhu L., Zhang C., Zhou J. Secreted frizzled-related protein 2 is epigenetically silenced and functions as a tumor suppressor in oral squamous cell carcinoma. Mol. Med. Rep. 2014; 10: 2293-2298.
49. Arun Gopinathan P., Kokila G., Jyothi M., Ananjan C., Pradeep L., Humaira S. Study of collagen birefringence in different grades of oral squamous cell carcinoma using picrosirius red and polarized light microscopy. Scientifica (Cairo). 2015; 2015:1-7.
50. Lapis K., Tímár J. Role of elastin matrix interactions in tumor progression. Semin Cancer Biol. 2002; 12: 209-217.
51. Sabnis S., Kulkarni M., Shinde S., Mani A. Morphological Changes Of Extracellular Matrix In Different Histopathological Grades Of Oral Squamous Cell Carcinoma. Annals of R.S.C.B. 2020; 24: 785-800.
52. Gobin E., Bagwell K., Wagner J., Mysona D., Sandirasegarane S., Smith N., Bai S., Sharma A., Schleifer R., She J.X. A pan-cancer perspective of matrix metalloproteases (MMP) gene expression profile and their diagnostic/prognostic potential. BMC Cancer. 2019; 19; 581.
53. Jose J., Heera R., Cherian L., Beena V., Paul S., Arun T.J. Evaluation of Connective Tissue Changes in Different Histological Grades of Oral Squamous Cell Carcinoma: A Histochemical Study. Oral & Maxillofacial Pathology Journal. 2023; 14: 12-17.
54. El-Kammar H., Afifi N.S., AbdulKhalik D. Role of Alpha Smooth Muscle Actin in Oral Squamous Cell Carcinoma Progression. EDJ. 2019; 65: 2387-2396.
55. Aziz N.C., Alahmad B.E., Kashmoola M.A., Lestari W., Mokhtar K.I., Rosdy N.M. Goniothalamus umbrosus Induces Cell Cycle Arrest in Oral Squamous Cell Carcinoma Cell Line. Journal of International Dental and Medical Research. 2024; 17: 996-999.