Scientific article
Volumen 36: Artículo x1y07135, 2025
e-ISSN 2215-3608, https://doi.org/10.15517/x1y07135
Soybean leaf infusion culture media and phytotoxic metabolite production in Macrophomina phaseolina*
Medio con infusión foliar de soja y producción de metabolitos fitotóxicos en Macrophomina phaseolina
Jazmín Vaceque-Acosta1, Javier E. Barúa1, Dani Daniel Ruiz-Diaz-Mendoza1, M. Cristina Romero-Rodríguez1, Antonio Macías-Sánchez2, María Eugenia Flores-Giubi1
* Reception: February 6, 2025. Acceptance: April 9, 2025. The work was part of a research project supported by the International Foundation for Science (IFS) [grant number IFS F/5783-1].
1 Universidad Nacional de Asunción, Facultad de Ciencias Químicas, Departamento de Química Biológica, San Lorenzo, Paraguay. jvaceque@qui.una.py (https://orcid.org/0009-0001-8577-902X); javierbarua@qui.una.py (https://orcid.org/0000-0002-8164-3432); druizdiaz@qui.una.py (https://orcid.org/0000-0001-9821-5656); rromero@qui.una.py (https://orcid.org/0000-0003-3979-0348); floresgiubi@qui.una.py (corresponding author; https://orcid.org/0000-0002-1572-9983).
2 Universidad de Cádiz, Facultad de Ciencias, Departamento de Química Orgánica, Cádiz, España. antoniojose.macias@gm.uca.es (https://orcid.org/0000-0001-6002-4977).
Abstract
Introduction. The charcoal rot fungus, Macrophomina phaseolina, is a ubiquitous necrotrophic phytopathogen that infecting soybean and other plant species. Despite its significant impact on crops, limited progress has been made in understanding the factors that influence phytotoxic molecule secretion by this phytopathogen. Objective. To evaluate the effect of soybean leaf infusion in the culture medium on the differential secretion of phytotoxic molecules of M. phaseolina. Materials and methods. The study was conducted between 2016 and 2023 at the Departamento de Química Biológica, Universidad Nacional de Asunción, Paraguay. Two fungal isolates were cultured in vitro using potato dextrose broth (PDB) and Czapek-Dox broth media, with or without soybean leaf infusion. Phytotoxic activity of secreted molecules was a using soybean leaf discs. The crude organic extract from the cultures was separated using chromatographic techniques, and purified metabolites were characterized by UHPLC-PDA/MS, HRMS (APGC), HRMSESI, 1HNMR and 13CNMR. Results. Molecules secreted by M. phaseolina FCQ11 cultured in infusion-enriched PDB induced the highest percentage of necrosis. Under these conditions, three differentially secreted metabolites were isolated and identified: (R)-mellein, (3R,4R)-hydroxymellein, and (-)-botryodiplodin. Conclusions. Soybean leaf infusion presence in M. phaseolina growth media stimulates phytotoxic metabolite production and alters the profile of secreted metabolites.
Keywords: fungal phytopathogen, secondary metabolites, bioactive molecules, charcoal rot.
Resumen
Introducción. El hongo de la pudrición carbonosa Macrophomina phaseolina es un fitopatógeno necrotrófico ubicuo que infecta a la soja y a otras especies de plantas. Aunque este hongo causa pérdidas significativas en los cultivos, se han logrado pocos avances en la comprensión de los factores que influyen en la secreción de moléculas fitotóxicas. Objetivo. Evaluar el efecto de la infusión de hojas de soja en el medio de cultivo sobre la secreción diferencial de moléculas fitotóxicas de M. phaseolina. Materiales y métodos. El estudio se realizó entre 2016 y 2023 en el Departamento de Química Biológica de la Universidad Nacional de Asunción, Paraguay. Dos aislados del hongo se cultivaron in vitro utilizando medios de caldo papa dextrosa y el medio Czapek-Dox, con o sin suplementación de infusión de hojas de soja. Se evaluó la actividad fitotóxica de las moléculas secretadas utilizando discos de hojas de soja. El extracto orgánico crudo de los cultivos se separó por medio de técnicas cromatográficas, y los metabolitos purificados se caracterizaron mediante UHPLC-PDA/MS, HRMS (APGC), HRMSESI, 1HNMR y 13CNMR. Resultados. Las moléculas secretadas por el hongo M. phaseolina FCQ11 cultivado en medio PDB enriquecido con infusión indujeron el mayor porcentaje de necrosis. Bajo estas condiciones, se aislaron e identificaron tres metabolitos secretados de forma diferencial: (R)-meleína, (3R,4R)-hidroximeleína y (-)-botriodiplodina. Conclusiones. La presencia de la infusión de hojas de soja en el medio de crecimiento de M. phaseolina estimula la producción de metabolitos fitotóxicos, alterando el perfil de los metabolitos secretados.
Palabras clave: hongo fitopatógeno, metabolitos secundarios, moléculas bioactivas, podredumbre carbonosa.
Introduction
The charcoal rot fungus Macrophomina phaseolina is a necrotrophic phytopathogen with a wide host range, including economically significant crops such as soybean (Glycine max L.) (Deshmukh & Tiwari, 2021), corn (Zea mays L.) (Saleh et al., 2010), and beans (Phaseolus vulgaris) (Marcenaro & Valkonen, 2016). It causes charcoal rot disease, which affects plants at all growth stages and is promoted by high temperatures and low soil moisture (Chilakala et al., 2022; Kaur et al., 2012; Khasin et al., 2021). Numerous factors contribute to the development of this pathology (Slavov, 2005), including phytotoxins, which facilitate the penetration, invasion, and colonization of the host (Kaur et al., 2012).
Several phytotoxins produced by M. phaseolina have been identified, including phaseolinone (Bhattacharya et al., 1987; Dhar et al., 1982), botryodiplodin (Ramezani et al., 2007; Salvatore et al., 2020), phaseocyclopentenones A and B, and Guignardone A (Masi et al., 2021). Other metabolites that may be related to the virulence of this pathogen, including phaseolinic acid (Mahato et al., 1987), macrophominol (Trigos et al., 1995), and mellein (Khambhati et al., 2023; Salvatore et al., 2020), have also been reported. However, many of the expected molecules from genome sequencing information are still undescribed, leaving a significant gap in understanding the role of these compounds in the pathogenesis of M. phaseolina (Islam et al., 2012).
Previous studies have shown that enriching the culture medium with host tissue can increase the secretion of phytotoxic molecules in phytopathogenic fungi such as Phytophthora capsici (Flores-Giubi et al., 2013) and, in the case of M. phaseolina, supplementing in vitro culture media with host plant infusions can stimulate conidia production (Nouri et al., 2020), alter the profile of secreted proteins (Pineda-Fretez et al., 2023) or modify the metabolite profile (Salvatore et al., 2020). Given the lack of reports on in vitro culture conditions that promote phytotoxin production in the soybean–M. phaseolina pathosystem and the fungus’s multifactorial pathogenicity, the objective of this research was to evaluate the effect of soybean leaf infusion in the culture medium on the differential secretion of phytotoxic molecules of M. phaseolina.
Materials and methods
General experimental procedures
The study was conducted from 2016 to 2023 at the Departamento de Química Biológica, Universidad Nacional de Asunción, Paraguay. NMR spectra were recorded on an Agilent 500 MHz NMR spectrometer using CDCl3 as the solvent (Eurisotop, Saint-Aubin, France). Chemical shifts are expressed in ppm (δ) and referenced to the solvent (δH 7.25, δC 77.0). Optical rotations were measured using an Anton PAAR 5300 polarimeter. Thin-layer chromatography (TLC) was performed on Merck Kieselgel Å F254 plates with a 0.25 mm layer thickness. Column chromatography was conducted on silica gel 60 (60−200 µm, VWR). For the ultra-high performance liquid chromatography (UHPLC) analysis, samples were injected into a Waters, Acquity™ UHPLC system coupled to a photodiode array (PDA) detector and a tandem mass spectrometer (Xevo TQD) operating in positive ion electrospray ionization mode (UHPLC/MS/ESI+).
The compounds [1] and [2] were analyzed using gas chromatography-atmospheric pressure chemical ionization coupled to high-resolution mass spectrometry (GC-APGC-HRMS) on an Agilent 7890 GC separation system, coupled with a quadrupole time-of-flight tandem mass spectrometer (Xevo-G2-S QTOF, Waters, Manchester, U.K.) equipped with an APGC source. Compound [3] was analyzed by high-resolution mass spectrometry with electrospray ionization (HRMSESI), into an ultra-high performance liquid chromatograph, coupled to a quadrupole time-of-flight tandem mass spectrometer (Xevo-G2-S QTOF, Waters, Manchester, U.K.) equipped with an ESI source. Data acquisition and processing for compounds [1-3] were performed with MassLynx™ 4.1 software (Waters, Manchester, U. K.) in positive ion mode.
Microorganisms
The fungal isolates M. phaseolina FCQ11 and FCQ39 were isolated from naturally infected soybean plants at Universidad Nacional de Asunción, San Lorenzo (Departamento Central), and Edelira (Departamento de Itapúa), Paraguay, respectively. Molecular identification was performed by Sanger sequencing of the translation elongation factor 1 alpha (tef1-α) and beta-tubulin (β-tub) genes at Macrogen Korea (Seoul, Republic of Korea). Sequences were deposited in GenBank under the following accession numbers: ON866534 (FCQ11-tef1-α), ON959212 (FCQ11-β-tub), ON866535 (FCQ39-tef1-α), and ON959213 (FCQ39-β-tub).
Culture conditions and fungal crude organic extracts preparation
M. phaseolina was grown and maintained on potato-dextrose-agar (PDA, Liofilchem™) in a microbiological incubator at 30 °C in the dark. Mycelium plugs (5 mm in diameter) were preserved in an 80 % glycerol aqueous solution at −20 °C. Stored mycelium plugs were re-inoculated on PDA culture medium and incubated at 30 °C, until the fungal mycelium covered the entire Petri dish surface (4-5 days).
Three fresh mycelium discs from actively growing margins were transferred to Roux bottles containing 200 mL of potato dextrose broth (PDB, Liofilchem™) or Czapek-Dox (CZP, Liofilchem™), both with soybean leaf infusion (PDB INF, CZP INF) or without soybean leaf infusion (PDB, CZP). The infusion was prepared by boiling 20 g of young soybean leaves (V3-V4 stage) in 1 L of distilled water, followed by filtration through gauze. The resulting infusion was then used to replace the water in the supplemented media.
After the fungal mycelium covered the entire culture surface (5-6 days), it was separated from the culture media by vacuum filtration using gauze. The filtrate obtained was extracted with ethyl acetate (HPLC grade), and the organic fraction was dried with anhydrous sodium sulfate and evaporated under reduced pressure using a rotary evaporator to yield crude organic extracts. The result was expressed as milligrams of organic extract per liter of culture medium (mg/L).
Phytotoxic bioassay
To evaluate the phytotoxic activity of crude organic extracts, bioassays were performed using 1.8 cm susceptible soybean cultivar Nidera A5009 (Glycine max, V3-V4 stage) leaf discs. Discs were placed on Petri dishes, and 50, 100, and 200 µg of each extract dissolved in acetonitrile were tested; 20 µL of 8 % (v/v) phosphoric acid (H3PO4) solution was used as a positive control and the solvent as a negative control (Moreau et al., 1982). The leaf discs were incubated in darkness at 30 °C for 24 hours. Following incubation, the presence of necrosis symptoms was observed. Total leaf area and leaf necrotic (damage) area of each disc were manually measured using ImageJ software (free license), and then the percentage of necrosis was then calculated (Schneider et al., 2012).
Isolation and identification of M. phaseolina metabolites
M. phaseolina FCQ11 grown in PDB INF was selected to perform the isolation of the molecules present in the crude organic extract and its subsequent identification. The crude organic extract was fractioned by column chromatography using silica gel as the stationary phase and a gradient of hexane and ethyl acetate with increasing polarity as the mobile phase. Fractions were collected in numbered test tubes, monitored by thin-layer chromatography (TLC), and grouped according to their retention factor (Rf) similarities. The metabolites were purified and identified by nuclear magnetic resonance (NMR) spectroscopy.
Compound 1 (R)-mellein
3,4-dihydro-8-hydroxy-3-methylisocoumarin. The organic extraction yield was 4.6 mg/100 mg of crude extract of M. phaseolina FCQ11 grown in PDB INF. The NMR spectroscopic data of compound 1 were consistent with the literature (Li et al., 2021). HRMS (APGC+) calcd for C10H11O3 [M+H]+ 179.0708, found 179.0728; calcd for C10H9O2 [M-H2O+H]+ 161.0603, found 161.0626; calcd for C9H11O2 [M-CO+H]+ 151.0759, found 151.0783; calcd for C9H9O [M-CO-H2O+H]+ 133.0653, found 133.0684.
Compound 2 (3R,4R)-hydroxymellein
(3R,4R)-4,8-dihydroxy-3-methylisochroman-1-one. The organic extraction yield was 7.2 mg/100 mg of crude extract of M. phaseolina FCQ11 grown in PDB INF. The NMR spectroscopic data of compound 2 were consistent with the literature (Djoukeng et al., 2009). HRMS (APGC+) calcd for C10H11O4 [M+H]+ 195.0657, found 195.0685; calcd for C10H9O3 [M-H2O+H]+ 177.0552, found 177.0583; calcd for C9H9O2 [M-H2O-CO+H]+ 149.0603, found 149.0627.
Compound 3 (-)-botryodiplodin
1-((3S,4R)-5-hydroxy-4-methyltetrahydrofuran-3-yl)ethan-1-one. The organic extraction yield was 9 mg/100 mg of crude extract of M. phaseolina FCQ11 grown in PDB INF. The optical rotation and NMR spectroscopic data of compound 3 matched the literature (Ramezani et al., 2007). HRMSESI m/z 167.0686 [M+Na]+ (calcd for C7H12O3Na, 167.0684), 145.0868 [M+H]+ (calcd for C7H13O3, 145.0865), 127.0762 [M+H-H2O]+ (calcd for C7H11O2, 127.0759).
Results
Total secondary metabolites secreted by M. phaseolina isolates
The production of secondary metabolites by M. phaseolina isolates FCQ11 and FCQ39 under different culture conditions was evaluated (Figures 1A and 1B for isolates FCQ11 and FCQ39, respectively). No differences were observed in the total production of secondary metabolites from M. phaseolina FCQ11 across the evaluated culture media. For M. phaseolina FCQ39, a lower production of secondary metabolites was detected in Czapek-Dox (CZP) medium without soybean leaf infusion (32.7 mg/L).
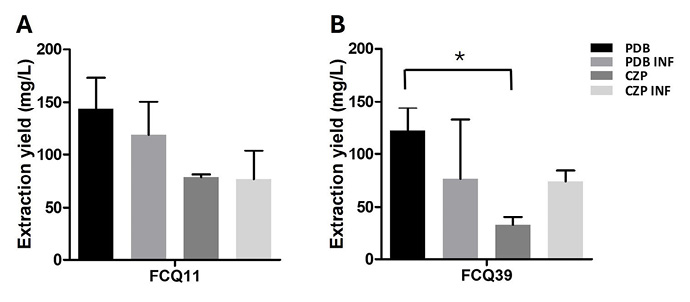
Figure 1. Secondary metabolites extraction yield from Macrophomina phaseolina FCQ11 (A) and FCQ39 (B). Departamento de Química Biológica, Facultad de Ciencias Químicas. Universidad Nacional de Asunción, Paraguay. 2016.
Mean organic extract yields (mg/L) was quantified from cultures grown in PDB and CZP media, with or without soybean leaf infusion (PDB INF and CZP INF, respectively). FCQ11 yields: 143.0mg/L (PDB), 118.6mg/L (PDB INF), 78.6mg/L (CZP), and 76.8mg/L (CZP INF). FCQ39 yields: 121.9mg/L (PDB), 76.6mg/L (PDB INF), 32.7mg/L (CZP), and 74.0mg/L (CZP INF). *p < 0.05 (ANOVA; Tukey).
Figura 1. Rendimiento de extracción de metabolitos secundarios de Macrophomina phaseolina FCQ11 (A) y FCQ39 (B). Departamento de Química Biológica, Facultad de Ciencias Químicas, Universidad Nacional de Asunción, Paraguay. 2016.
El rendimiento promedio de los extractos orgánicos (mg/L) se determinó a partir de cultivos en medios PDB y CZP, con o sin infusión de hojas de soja (PDB INF y CZP INF, respectivamente). Para FCQ11, los rendimientos fueron 143,0 mg/L (PDB), 118,6 mg/L (PDB INF), 78,6 mg/L (CZP) y 76,8 mg/L (CZP INF). Para FCQ39, los rendimientos fueron 121,9 mg/L (PDB), 76,6 mg/L (PDB INF), 32,7 mg/L (CZP) y 74,0 mg/L (CZP INF). *p < 0,05 (ANOVA; Tukey).
Phytotoxicity of metabolites secreted by M. phaseolina
The bioassay on soybean leaf discs revealed clear symptoms of tissue damage and visible necrosis, confirming that both M. phaseolina isolates produce phytotoxic compounds capable of inducing cell death in soybean tissues. Representative images and results of the bioassay are shown in Figure 2A. For both isolates, a 100 % necrotic effect was observed with 100 and 200 µg of crude organic extract from all media tested. However, with a smaller amount of crude organic extract (50 µg), statistically significant differences were found, with the crude organic extract of M. phaseolina FCQ11 grown in PDB INF medium showing the highest foliar damage (Figure 2B). This extract was chosen for metabolites isolation and purification.
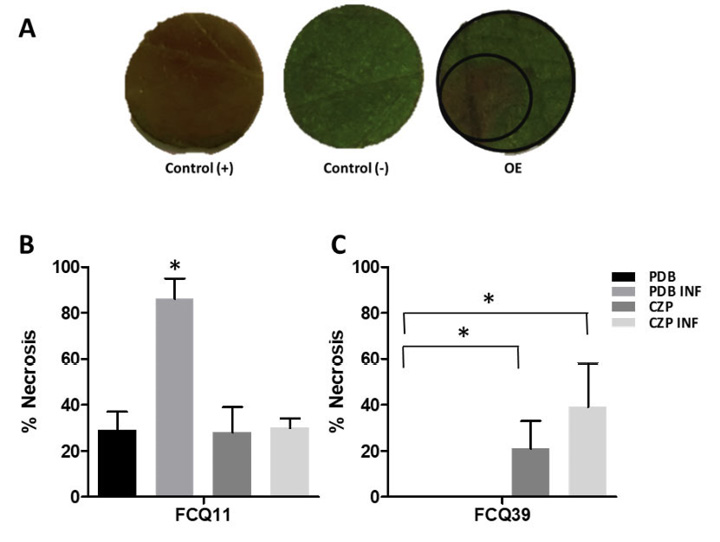
Figure 2. Phytotoxicity bioassay results. A. Representative images showing phytotoxic activity of metabolites secreted by Macrophomina phaseolina FCQ11 on soy leaves discs. Control (+): H3PO4 solution as positive control; Control (−),]: acetonitrile as negative control; OE: crude organic extract from PDB INF tested at 50 μg. Departamento de Química Biológica, Facultad de Ciencias Químicas, Universidad Nacional de Asunción, Paraguay. 2016.
The outer circle demarcates the total leaf area while the inner circle indicates the necrotic region; results are expressed as percentage of leaf area with signs of necrosis,m analyzed using ImageJ software. B, C. Necrosis percentage induced by secreted molecules from M. phaseolina FCQ11 and FCQ39 (B and C, respectively). Crude organic extracts of PDB and PDB-INF from M. phaseolina FCQ39 showed no phytotoxic activity. For each assessment, crude organic extracts were obtained from M. phaseolina filtrates grown in potato dextrose broth (PDB), PDB with soy leaves media infusion (PDB INF), Czapek-Dox (CZP), and Czapek-Dox with soy leaves media infusion (CZP INF), evaluated at 50 μg. *p < 0.05 (ANOVA; Tukey).
Figura 2. Bioensayo de fitotoxicidad. A. Imágenes representativas de la actividad fitotóxica de los metabolitos secretados por Macrophomina phaseolina FCQ11 sobre discos de hojas de soja. Control (+), control positivo solución H3PO4; Control (−), control negativo acetonitrilo; OE, extracto orgánico crudo de PDB INF evaluado a 50 μg. Departamento de Química Biológica, Facultad de Ciencias Químicas, Universidad Nacional de Asunción, Paraguay. 2016.
El círculo mayor indica el área foliar total y el círculo menor el área de necrosis detectada; los resultados se expresaron como porcentaje del área foliar con signos de necrosis. El análisis se realizó con el programa informático ImageJ. B, C. Porcentaje de necrosis inducida por moléculas secretadas de M. phaseolina FCQ11 y FCQ39 (B y C, respectivamente). Los extractos orgánicos crudos de PDB y PDB INF de M. phaseolina FCQ39 no mostraron actividad fitotóxica. En cada caso, el extracto orgánico crudo se obtuvo del filtrado de M. phaseolina cultivado en medios caldo papa dextrosa (PDB), PDB con infusión de hojas de soja (PDB INF), Czapek-Dox (CZP), Czapek-Dox con infusión de hojas de soja (CZP INF), evaluado a 50 μg. *p < 0,05 (ANOVA; Tukey).
Isolation and identification of metabolites secreted by M. phaseolina
Ten fractions were obtained from the organic extract of M. phaseolina FCQ11. Among them, the metabolites were identified, isolated, and later subjected to structural characterization. Three main metabolites as phytotoxic molecules secreted by M. phaseolina were detected and isolated: (R)-mellein [1], (3R,4R)-hydroxymellein [2], and (-)-botryodiplodin [3] (Figure 3).
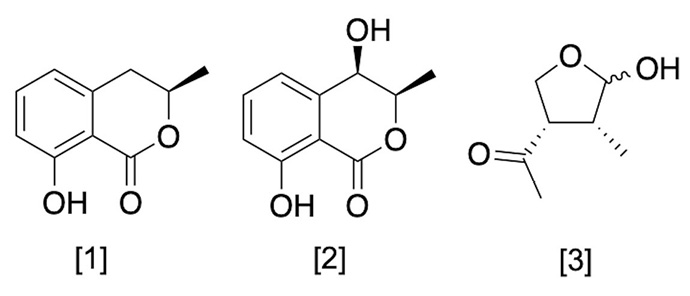
Figure 3. Metabolites structure secreted by Macrophomina phaseolina. Departamento de Química Biológica, Facultad de Ciencias Químicas, Universidad Nacional de Asunción, Paraguay. 2023.
Figura 3. Estructura de los metabolitos secretados por Macrophomina phaseolina. Departamento de Química Biológica, Facultad de Ciencias Químicas, Universidad Nacional de Asunción, Paraguay. 2023.
The optical rotation and nuclear magnetic resonance (NMR) spectroscopic data of the pure compounds (R)-mellein [1] and (3R,4R)-hydroxymellein [2] matched those previously described in the literature. A third metabolite [3] was isolated and analyzed by UHPLC/PDA. Despite its low UV absorbance, high-resolution mass spectrometry confirmed the molecular weight of the metabolite with a retention time of 0.48 min at 145.0868 [M+H]+, which was consistent with the data published in the literature for (-)-botryodiplodin.
The chromatographic profiles of the crude organic extracts from M. phaseolina FCQ11 grown in PDB and PDB INF revealed distinct patterns of secreted metabolites (Figure 4). The major metabolites identified were (R)-mellein and (3R,4R)-hydroxymellein, with retention times of 2.03 and 1.08 min, respectively. The metabolite (-)-botryodiplodin was detected as a minority peak at 0.48 min. All metabolites were identified by mass spectrometry by comparison with UHPLC-MS analysis.
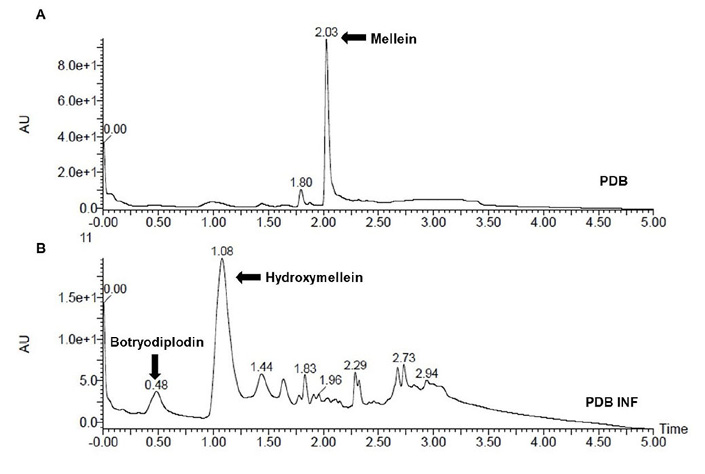
Figure 4. Chromatographic fingerprint by ultra-high efficacy liquid chromatography with diode array detection (UHPLC-PDA) of secondary metabolites secreted by Macrophomina phaseolina FCQ11. A. Fungus grew in potato dextrose broth without soybean leaf infusion (PDB). B. Fungus grew in potato dextrose broth with soybean leaf infusion (PDB INF). Departamento de Química Biológica, Facultad de Ciencias Químicas, Universidad Nacional de Asunción, Paraguay. 2016.
Figura 4. Cromatograma obtenido por cromatografía líquida de ultra alta eficacia con detección por arreglo de diodos (UHPLC-PDA) de metabolitos secundarios secretados por Macrophomina phaseolina FCQ11. A. El hongo fue cultivado en caldo papa dextrosa sin infusión de hojas de soja (PDB). B. El hongo fue cultivado en caldo papa dextrosa con infusión de hojas de soja (PDB INF). Departamento de Química Biológica, Facultad de Ciencias Químicas, Universidad Nacional de Asunción, Paraguay. 2016.
The chromatographic analysis showed that the addition of soybean leaf infusion to the PDB culture medium of M. phaseolina FCQ11 altered the chemical composition of the medium. When M. phaseolina FCQ11 was grown only in PDB, the secretion of (R)-mellein was observed (Figure 4A). However, when a soybean leaf infusion was added, the secretion of (3R,4R)-hydroxymellein and (-)-botryodiplodin was detected, along with other molecules in a lower proportion (Figure 4B).
Discussion
M. phaseolina, the causal agent of charcoal rot, infects soybean plants at any crop growth stage, although post-flowering is the most common (Mishra & Kumari, 2021). As soybean is a key agricultural crop, infections caused by this phytopathogenic fungus highlight the importance of understanding the molecular mechanisms underlying its pathogenicity. This soil-borne pathogen has a high genomic potential for secreting low molecular weight molecules that could be involved in the infection process of its host plants (Islam et al., 2012).
Research has focused on detecting molecules related to pathogenesis, revealing significant differences in metabolic profiles when the fungus is grown under pathogenesis-simulating conditions. For example, in Phytophthora capsici, habanero pepper leaf infusion was used to supplement the medium, enhancing the understanding of its pathogenic behavior (Flores-Giubi et al., 2013). The fungus M. phaseolina showed significant changes in metabolite profiles when grown in media supplemented with Eucalyptus globulus stem tissues (Salvatore et al., 2020). Supplementation with pistachio leaf extract was shown to affect conidia production of M. phaseolina (Nouri et al., 2020). More recently, soybean leaf infusion was found to induce significant alterations in the fungal secretome of M. phaseolina (Pineda-Fretez et al., 2023).
The metabolites (R)-mellein [1] and (3R,4R)-hydroxymellein [2], secreted by M. phaseolina, were identified through spectroscopic techniques. The data obtained were consistent with previously published data from NMR, optical rotation, and mass spectrometry (Djoukeng et al., 2009; Li et al., 2021). Spectrometric analysis revealed characteristic fragmentation patterns of protonated molecular ions, which were in agreement with the assigned structures (Félix et al., 2019; Khambhati et al., 2023).
Mellein, the main chemical structure from the 3,4-dihydroisocoumarins group, is widely found in different fungi in both R or S form at C-3, with the R form being more common (Braca et al., 2012). The first report of (R)-mellein was isolated from Aspergillus melleus (Nishikawa, 1933). It has been described as a phytotoxic compound, causing symptoms such as wilting, chlorosis, and necrosis in pine seedlings, tomato cuttings, grapevines, and others (Abou-Mansour et al., 2015; Cabras et al., 2006; Saeed, 2016). A complete inhibition of wheat seed (Triticum aestivum) germination has been observed, and it has also been seen in vine seedlings. In in vitro assays, (R)-mellein strongly suppresses the expression of plant defense genes and can accumulate in plants in its native chemical form (Chooi et al., 2015; Trotel-Aziz et al., 2019).
The metabolite (3R,4R)-hydroxymellein, a derivative of (R)-mellein, has several structural isomers (Cabras et al., 2006). It is also considered a phytotoxic metabolite in different fungi, and it is believed that it can generate synergy or addition with other secreted metabolites, thus affecting susceptible plants or even acting alone. However, its phytotoxicity depends on multiple factors, such as the host species and assay conditions used to evaluate it (Abou-Mansour et al., 2015; Cabras et al., 2006), or the conditions to which the fungus is exposed (Pan et al., 2019; Ramírez-Suero et al., 2014; Trotel-Aziz et al., 2019).
Botryodiplodin, a phytotoxin initially described in Penicillium roqueforti (Moreau et al., 1982; Nielsen et al., 2006; Renauld et al., 1985) and P. stipitatum (Fuska et al., 1974; 1988), has also been reported in M. phaseolina. It is believed that this fungus utilizes (-)-botryodiplodin during root infection to induce necrosis in root tissue, facilitating the entry of fungal hyphae into the plant (Abbas et al., 2019; Islam et al., 2012; Ramezani et al., 2007). The metabolites identified in this study could be part of the strategy used by this fungus in the process of interaction with the host, making their analysis crucial for understanding the fungus-host interaction mechanisms (Reveglia et al., 2020).
Although these metabolites have been previously documented, the specific conditions for in vitro secretion by M. phaseolina have not been described. The addition of soybean leaf infusion to the culture medium was the key to inducing the production of these metabolites. In addition to the three molecules detected, other secreted molecules, albeit in smaller amounts (Figure 4B), may also have been responsible for the high phytotoxicity observed in the secreted metabolites of strain FCQ11 in the medium enriched with soybean leaf infusion. Further research is underway to identify the molecules and clarify their specific roles.
Conclusions
The inclusion of soybean leaf infusion in the growth medium of M. phaseolina promoted the production of phytotoxic metabolites in a potato dextrose broth medium supplemented with soybean leaf infusion (PDB INF), and under this condition, the profile of secreted metabolites changed. Three metabolites —(R)-mellein [1], (3R,4R)-hydroxymellein [2], and (-)-botryodiplodin [3]— were identified, and these could be related to the symptoms developed in susceptible soybean crops. The findings of this study provide new insights into the mechanisms by which this necrotrophic fungus infects susceptible hosts.
Acknowledgements
The authors are grateful to the Departamento de Botánica, FCQ-UNA, Paraguay, for providing the facilities for the acclimatization of soybean plants. Thanks are also extended to Isidro G. Collado and Rosario Hernández-Galán from Universidad de Cádiz, Spain, for their invaluable support in the isolation and identification of metabolites, and to Eliane Oliveira Silva from the Federal University of Bahia, Brazil, and Carlos Benitez, Paraguay, for the technical assistance with the optical rotation measurements.
Interests conflict
The authors declare that there are no conflicts of interest regarding the publication of this article.
References
Abbas, H. K., Bellaloui, N., Accinelli, C., Smith, J. R., & Thomas Shier, W. (2019). Toxin production in soybean (Glycine max L.) plants with charcoal rot disease and by Macrophomina phaseolina, the fungus that causes the disease. Toxins, 11(11), Article 645. https://doi.org/10.3390/toxins11110645
Abou-Mansour, E., Débieux, J.-L., Ramírez-Suero, M., Bénard-Gellon, M., Magnin-Robert, M., Spagnolo, A., Chong, J., Farine, S., Bertsch, C., L’Haridon, F., Serrano, M., Fontaine, F., Rego, C., & Larignon, P. (2015). Phytotoxic metabolites from Neofusicoccum parvum, a pathogen of Botryosphaeria dieback of grapevine. Phytochemistry, 115, 207–215. https://doi.org/10.1016/j.phytochem.2015.01.012
Bhattacharya, G., Dhar, T. K., Bhattacharya, K., & Siddiqui, K. A. I. (1987). Mutagenic action of phaseolinone, a mycotoxin isolated from Macrophomina phaseolina. Australian Journal of Biological Sciences, 40(4), 349–354. https://doi.org/10.1071/BI9870349
Braca, A., Bader, A., & De Tommasi, N. (2012). Plant and fungi 3,4-Dihydroisocoumarins: structures, biological activity, and taxonomic relationships. In R. Atta-ur (Ed.), Studies in natural products chemistry (pp. 191–215). Elsevier. https://doi.org/10.1016/B978-0-444-59514-0.00007-9
Cabras, A., Mannoni, M. A., Serra, S., Andolfi, A., Fiore, M., & Evidente, A. (2006). Occurrence, isolation and biological activity of phytotoxic metabolites produced in vitro by Sphaeropsis sapinea, pathogenic fungus of Pinus radiata. European Journal of Plant Pathology, 115(2), 187–193. https://doi.org/10.1007/s10658-006-9006-7
Chilakala, A. R., Mali, K. V., Irulappan, V., Patil, B. S., Pandey, P., Rangappa, K., Ramegowda, V., Kumar, M. N., Puli, C. O. R., Mohan-Raju, B., & Senthil-Kumar, M. (2022). Combined Drought and Heat Stress Influences the Root Water Relation and Determine the Dry Root Rot Disease Development Under Field Conditions: A Study Using Contrasting Chickpea Genotypes. Frontiers in Plant Science, 13, Article 890551. https://doi.org/10.3389/fpls.2022.890551
Chooi, Y. H., Krill, C., Barrow, R. A., Chen, S., Trengove, R., Oliver, R. P., & Solomon, P. S. (2015). An In planta-expressed polyketide synthase produces (R)-mellein in the wheat pathogen Parastagonospora nodorum. Applied and Environmental Microbiology, 81(1), 177–186. https://doi.org/10.1128/AEM.02745-14
Deshmukh, R., & Tiwari, S. (2021). Molecular interaction of charcoal rot pathogenesis in soybean: a complex interaction. Plant Cell Reports, 40, 1799–1812. https://doi.org/10.1007/s00299-021-02747-9
Dhar, T. K., Siddiqui, K. A. I., & Ali, E. (1982). Structure of phaseolinone, a novel phytotoxin from Macrophomina phaseolina. Tetrahedron Letters, 23(51), 5459–5462. https://doi.org/10.1016/0040-4039(82)80157-3
Djoukeng, J. D., Polli, S., Larignon, P., & Abou-Mansour, E. (2009). Identification of phytotoxins from Botryosphaeria obtusa, a pathogen of black dead arm disease of grapevine. European Journal of Plant Pathology, 124, 303–308. https://doi.org/10.1007/s10658-008-9419-6
Félix, C., Salvatore, M. M., DellaGreca, M., Ferreira, V., Duarte, A. S., Salvatore, F., Naviglio, D., Gallo, M., Alves, A., Esteves, A. C., & Andolfi, A. (2019). Secondary metabolites produced by grapevine strains of Lasiodiplodia theobromae grown at two different temperatures. Mycologia, 111(3), 466–476. https://doi.org/10.1080/00275514.2019.1600342
Flores-Giubi, M. E., Brito-Argáez, L., García-Sosa, K., Escalante-Erosa, F., Islas-Flores, I., & Peña-Rodríguez, L. M. (2013). Optimization of culturing conditions of a strain of Phytophthora capsici pathogenic to habanero pepper (Capsicum chinense). Journal of Phytopathology, 161(11–12), 807–813. https://doi.org/10.1111/jph.12138
Fuska, J., Nemec, P., Fuskova, A., & Kuhr, I. (1974). Antitumor antibiotics produced by Penicillium stipitatum thom. The Journal of Antibiotics, 27(2), 123–127. https://doi.org/10.7164/antibiotics.27.123
Fuska, J., Proksa, B., & Uhrín, D. (1988). The antibiotic PSX-1 Produced by Pennicilium stipitatum is identical with botryodiplodin. Folia Microbiologica, 33(3), 238–240. https://doi.org/10.1007/BF02925912
Islam, M. S., Haque, M. S., Islam, M. M., Emdad, E. M., Halim, A., Hossen, Q. M. M., Hossain, M. Z., Ahmed, B., Rahim, S., Rahman, M. S., Alam, M. M., Hou, S., Wan, X., Saito, J. A., & Alam, M. (2012). Tools to kill: Genome of one of the most destructive plant pathogenic fungi Macrophomina phaseolina. BMC Genomics, 13(1), Article 493. https://doi.org/10.1186/1471-2164-13-493
Kaur, S., Dhillon, G. S., Brar, S. K., Vallad, G. E., Chand, R., & Chauhan, V. B. (2012). Emerging phytopathogen Macrophomina phaseolina: biology, economic importance and current diagnostic trends. Critical Reviews in Microbiology, 38(2), 136–151. https://doi.org/10.3109/1040841X.2011.640977
Khambhati, V. H., Abbas, H. K., Sulyok, M., Tomaso-Peterson, M., Chen, J., & Shier, W. T. (2023). Mellein: Production in culture by Macrophomina phaseolina isolates from soybean plants exhibiting symptoms of charcoal rot and its role in pathology. Frontiers in Plant Science, 14, Article 1105590. https://doi.org/10.3389/fpls.2023.1105590
Khasin, M., Bernhardson, L. F., O’Neill, P. M., Palmer, N. A., Scully, E. D., Sattler, S. E., & Funnell-Harris, D. L. (2021). Pathogen and drought stress affect cell wall and phytohormone signaling to shape host responses in a sorghum COMT bmr12 mutant. BMC Plant Biology, 21, Article 391. https://doi.org/10.1186/s12870-021-03149-5
Li, Y., Gai, Z., Wang, C., Li, P., & Li, B. (2021). Identification of mellein as a pathogenic substance of Botryosphaeria dothidea by UPLC-MS/MS analysis and phytotoxic bioassay. Journal of Agricultural and Food Chemistry, 69(30), 8471–8481. https://doi.org/10.1021/acs.jafc.1c03249
Mahato, S. B., Siddiqui, K. A. I., Bhattacharya, G., Ghosal, T., Miyahara, K., Sholichin, M., & Kawasaki, T. (1987). Structure and stereochemistry of phaseolinic acid: a new acid from Macrophomina phaseolina. Journal of Natural Products, 50(2), 245–247. https://doi.org/10.1021/np50050a024
Marcenaro, D., & Valkonen, J. P. T. (2016). Seedborne pathogenic fungi in common bean (Phaseolus vulgaris cv. INTA rojo) in Nicaragua. PLoS ONE, 11(12), Article e0168662. https://doi.org/10.1371/journal.pone.0168662
Masi, M., Sautua, F., Zatout, R., Castaldi, S., Arrico, L., Isticato, R., Pescitelli, G., Carmona, M. A., & Evidente, A. (2021). Phaseocyclopentenones A and B, phytotoxic penta- and tetrasubstituted cyclopentenones produced by Macrophomina phaseolina, the causal agent of charcoal rot of soybean in argentina. Journal of Natural Products, 84(2), 459–465. https://doi.org/10.1021/acs.jnatprod.0c01287
Mishra, P. K. & Kumari, M., (2021). Morpho-cultural and pathogenic variability in Macrophomina phaseolina isolates from soybean. The Pharma Innovation Journal, 10(3), 777–785.
Moreau, S., Biguet, J., Lablache-Combier, A., Foulon, C., & Delfosse, M. (1982). Botryodiplodin, a mycotoxin synthesized by a strain of P. roqueforti. Journal of Organic Chemistry, 47(12), 2358–2359. https://doi.org/10.1021/jo00133a024
Nielsen, K. F., Sumarah, M. W., Frisvad, J. C., & Miller, J. D. (2006). Production of metabolites from the Penicillium roqueforti complex. Journal of Agricultural and Food Chemistry, 54(14), Article 5216. https://doi.org/10.1021/jf0680081
Nishikawa, H. (1933). Biochemistry of filamentous fungi. II: A metabolic product of Aspergillus melleus Yukawa. Part I. Bulletin of the Agricultural Chemical Society of Japan, 9(7-9), 107-109. https://doi.org/10.1080/03758397.1933.10857042
Nouri, M. T., Lawrence, D. P., Kallsen, C. E., & Trouillas, F. P. (2020). Macrophomina crown and root rot of pistachio in California. Plants, 9(2), Article 134. https://doi.org/10.3390/plants9020134
Pan, R., Bai, X., Chen, J., Zhang, H., & Wang, H. (2019). Exploring structural diversity of microbe secondary metabolites using OSMAC strategy: a literature review. Frontiers in Microbiology, 10, Article 294. https://doi.org/10.3389/fmicb.2019.00294
Pineda-Fretez, A., Orrego, A., Iehisa, J. C. M., Flores-Giubi, M. E., Barúa, J. E., Sánchez-Lucas, R., Jorrín-Novo, J., & Romero-Rodríguez, M. C. (2023). Secretome analysis of the phytopathogen Macrophomina phaseolina cultivated in liquid medium supplemented with and without soybean leaf infusion. Fungal Biology, 127(5), 1043–1052. https://doi.org/10.1016/J.FUNBIO.2023.04.001
Ramezani, M., Shier, W. T., Abbas, H. K., Tonos, J. L., Baird, R. E., & Sciumbato, G. L. (2007). Soybean charcoal rot disease fungus Macrophomina phaseolina in Mississippi produces the phytotoxin (-)-botryodiplodin but no detectable phaseolinone. Journal of Natural Products, 70(1), 128–129. https://doi.org/10.1021/np060480t
Ramírez-Suero, M., Bénard-Gellon, M., Chong, J., Laloue, H., Stempien, E., Abou-Mansour, E., Fontaine, F., Larignon, P., Mazet-Kieffer, F., Farine, S., & Bertsch, C. (2014). Extracellular compounds produced by fungi associated with Botryosphaeria dieback induce differential defence gene expression patterns and necrosis in Vitis vinifera cv. Chardonnay cells. Protoplasma, 251(6), 1417–1426. https://doi.org/10.1007/s00709-014-0643-y
Renauld, F., Moreau, S., Lablache-Combier, A., & Tiffon, B. (1985). Botryodiplodin: A mycotoxin from Penicillium roqueforti: reaction with amino-pyrimidines, amino-purines and 2’-deoxynucleosides. Tetrahedron, 41(5), 955–962. https://doi.org/10.1016/S0040-4020(01)96415-4
Reveglia, P., Masi, M., & Evidente, A. (2020). Melleins — Intriguing Natural Compounds. Biomolecules, 10(5), Article 772. https://doi.org/10.3390/biom10050772
Saeed, A. (2016). Isocoumarins, miraculous natural products blessed with diverse pharmacological activities. European Journal of Medicinal Chemistry, 116, 290–317. https://doi.org/10.1016/j.ejmech.2016.03.025
Saleh, A. A., Ahmed, H. U., Todd, T. C., Travers, S. E., Zeller, K. A., Leslie, J. F., & Garrett, K. A. (2010). Relatedness of Macrophomina phaseolina isolates from tallgrass prairie, maize, soybean and sorghum. Molecular Ecology, 19(1), 79–91. https://doi.org/10.1111/j.1365-294X.2009.04433.x
Salvatore, M. M., Félix, C., Lima, F., Ferreira, V., Naviglio, D., Salvatore, F., Duarte, A. S., Alves, A., Andolfi, A., & Esteves, A. C. (2020). Secondary metabolites produced by Macrophomina phaseolina isolated from Eucalyptus globulus. Agriculture, 10(3), Article 72. https://doi.org/10.3390/agriculture10030072
Schneider, C. A., Rasband, W. S., Eliceiri, K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nature Methods, 9, 671-675. https://doi.org/10.1038/nmeth.2089
Slavov, S. (2005). Phytotoxins and in vitro screening for improved disease resistant plants. Biotechnology & Biotechnological Equipment, 19(Suppl. 3), 48–55. https://doi.org/10.1080/13102818.2005.10817285
Trigos, A., Reyna, S. & Matamoros, B. (1995). Macrophominol a diketopiperazine from cultures of Macrophomina phaseolina. Phytochemistry, 40(6), 1697–1698. https://doi.org/10.1016/0031-9422(95)00626-I
Trotel-Aziz, P., Abou-Mansour, E., Courteaux, B., Rabenoelina, F., Clément, C., Fontaine, F., & Aziz, A. (2019). Bacillus subtilis PTA-271 counteracts Botryosphaeria dieback in grapevine, triggering immune responses and detoxification of fungal phytotoxins. Frontiers in Plant Science, 10, Article 25. https://doi.org/10.3389/fpls.2019.00025

Esta obra está bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional.
Puede hallar permisos más allá de los concedidos con esta licencia en pccmca@gmail.com