Resumen
En diciembre del 2019, surgió una nueva enfermedad por coronavirus (COVID-19) que se presentó como una neumonía con etiología desconocida en Wuhan, China. La propagación continua de COVID-19 en todo el mundo está creando una enorme incertidumbre. En particular, la profesión dental se enfrenta a un gran desafío. Debido a las características de los consultorios dentales, el riesgo de infección cruzada es elevado entre los pacientes y el personal. La presente revisión tuvo como objetivo discutir brevemente la visión general de la enfermedad, incluyendo conceptos de ciencia básica, la epidemiología, vías de transmisión, síntomas y varios temas que los autores consideran vinculantes y relevantes para la práctica dental. Las directrices adoptadas por los gobiernos y las organizaciones internacionales también involucran a todas las asociaciones dentales para proteger la salud de la comunidad y contener la propagación de la infección por COVID-19 hasta que una vacuna o un tratamiento eficaz/validado esté disponible.
Citas
World Health Organization, WHO Director-General’s Remarks at the Media Briefing on 2019-nCoV on 11 February 2020; 2020 [cited 2020 Feb 10]. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020
Organización Mundial de la Salud. Declaración sobre la segunda reunión del Comité de Emergencias del Reglamento Sanitario Internacional (2005) acerca del brote del nuevo coronavirus (2019-nCoV); [cited 2020 Feb 1]. Available from: https://www.who.int/es/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov)
Organización Mundial de la Salud. Alocución de apertura del Director General de la OMS en la rueda de prensa sobre la COVID-19 celebrada el 11 de marzo de 2020; [cited 2020 Mar 11]. Available from: https://www.who.int/es/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J., Liu W., Bi Y., Gao G. F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016; 24 (6): 490-502.
Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016; 3 (1): 237-61.
Wu A.., Peng Y., Huang B., Ding X., Wang X., Niu P., Meng J., Zhu Z., Zhang Z., Wang J., Sheng J., Quan L., Xia Z., Tan W., Cheng G., Jiang T. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020; 27 (3): 325-28.
Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G. F., Tan W.; China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382 (8): 727-33.
Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020; 5 (4): 536-44.
Sohrabi C., Alsafi Z., O'Neill N., Khan M., Kerwan A., Al-Jabir A., Iosifidis C., Agha R.. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int J Surg. 2020; 76: 71-76.
Fehr A. R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015; 1282: 1-23.
Mousavizadeh L., Ghasemi S. Genotype and phenotype of COVID-19: Their roles in pathogenesis [published online ahead of print, 2020 Mar 31]. J Microbiol Immunol Infect. 2020; S1684-1182 (20) 30082-7.
Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X1, Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W. J., Wang D., Xu W., Holmes E. C., Gao G. F., Wu G., Chen W., Shi W., Tan W. Genomic characterization and epidemiology of 2019 novel corona virus: implications for virus origins and receptor binding. Lancet 2020; 395 (10224): 565-74.
Paraskevis O., Kostaki E. G., Magiorkinis G., Panayiotakopoulos G., Sourvinos G., Tsiodras S.. Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. lnfect Genet Evol. 2020; 79: 104212.
Sahu K. K., Mishra A. K., Lal A. COVID-2019: update on epidemiology, disease spread and management. Monaldi Arch Chest Dis. 2020; 90 (1). doi:10.4081/monaldi.2020.1292
Wan Y., Shang J., Graham R., Baric R. S., Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J Virol 2020; 94 (7): pii: e00127-20.
Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T. S., Herrler G., Wu N. H., Nitsche A., Müller M. A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020; 181 (2): 271-80.e8.
Guo Y-R, Cao Q-D, Hong Z-S, Tan Y-Y, Chen S-D, Jin H-J, Tan KS, Wang DY, Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak an update on the status. Mil Med Res 2020; 7 (1): 1e10.
Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K. S. M., Lau E. H. Y., Wong J. Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T. T. Y., Wu J. T., Gao G. F. L., Cowling B. J., Yang B., Leung G. M., Feng Z. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020; 382 (13): 1199-1207.
Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J., Guo Q., Song T., He J., Yen H. L., Peiris M., Wu J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020; 382 (12): 1177-79.
Ather A., Patel B., Ruparel N. B., Diogenes A., Hargreaves K. M. Coronavirus disease 19 (COVID-19): implications for clinical Dental Care. J Endod. 2020; 46 (5): 584-95.
Lui, S. et al. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int J Surg. 2020; 76: 71-76.
Hagen, A. COVID-19 transmission dynamics. American Society for Microbiology; 2020 [cited 2020 Apr 23]. Available from: https://stage.asm.org/Articles/2020/April/COVID-19-Transmission-Dynamics
Meng L., Hua F., Bian Z. Coronavirus disease 2019 (COVID-19): emerging and future challenges for dental and oral medicine. J Dent Res. 2020; 99 (5): 481-87.
Spagnuolo G., De Vito D., Rengo S., Tatullo M. COVID-19 outbreak: an overview on dentistry. Int J Environ Res Public Health. 2020; 17 (6): 2094.
To K. K., Tsang O. T., Chik-Yan Yip C., Chan K. H., Wu T. C., Chan J. M. C., Leung W. S., Chik T. S., Choi C. Y., Kandamby D. H., Lung D. C., Tam A. R., Poon R. W., Fung A. Y., Hung I. F., Cheng V. C., Chan J. F., Yuen K. Y. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020;ciaa149. doi: 10.1093/cid/ciaa149
Sabino-Silva R., Jardim A. C. G., Siqueira W. L. Coronavirus COVID-19 impacts to dentistry and potential salivary diagnosis. Clin Oral Investig. 2020; 24 (4): 1619-21.
Peng X., Xu X., Li Y., Cheng L., Zhou X., Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci. 2020; 12 (1): 9.
Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings; 2020 [cited 2020 April 24]. Available at: https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html
Centers for Disease Control and Prevention. Interim infection prevention and control guidance for dental settings during the COVID-19 response. 2020 [cited 2020 Apr 23]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/dental-settings.html
American Dental Association. ADA Interim guidance for minimizing risk of COVID-19 transmission; 2020 [cited 2020 April 20]. Available at: https://www.ada.org/~/media/CPS/Files/COVID/ADA_COVID_Int_Guidance_Treat_Pts
Ministerio de Salud de Costa Rica, Caja Costarricense de Seguro Social, INCIENSA, OPS. Lineamientos Nacionales para la Vigilancia de la enfermedad COVID-19 Costa Rica Versión N ° 7. 2020 [cited 2020 Apr 23]. Available from: https://www.ministeriodesalud.go.cr/sobre_ministerio/prensa/docs/version_7_lineamientos_nacionales_vigilancia_infeccion_coronavirus_11032020.pdf.
American Dental Association. ADA interim guidance for management of emergency and urgent dental care; 2020 [ cited 2020 Apr 20]. Available from: https://www.ada.org/~/media/CPS/Files/COVID/ADA_Int_Guidance_Mgmt_Emerg-Urg_Dental_COVID19.pdf
Centers for Disease Control and Prevention. Summary of infection prevention practices in dental settings basic expectations; 2016 [cited 2020 Apr 25]. Available from: https://www.cdc.gov/oralhealth/infectioncontrol/pdf/safe-care2.pdf
World Health Organization (WHO). Rational use of personal protective equipment for coronavirus disease (COVID-19) and considerations during severe shortages; 2020 [cited 2020 Apr 26]. Available from: https://www.who.int/publications-detail/rational-use-of-personal-protective-equipment-for-coronavirus-disease-(covid-19)-and-considerations-during-severe-shortages.
Centre for Disease Control and Prevention. Use personal protective equipment (PPE) when caring for patients with confirmed or suspected COVID-19; 2020 [cited 2020 Apr 23]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/downloads/A_FS_HCP_COVID19_PPE.pdf
Alharbi A., Alharbi S., Alqaidi S. Guidelines for dental care provision during the COVID-19 pandemic. Saudi Dent J. doi: 10.1016/j.sdentj.2020.04.001
Center for Disease Control and Prevention. Interim U.S. guidance for risk assessment and public health management of healthcare personnel with potential exposure in a healthcare setting to patients with coronavirus disease 2019 (COVID-19); 2020 [cited 2020 Apr 24]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html
World Health Organization. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations; 2020 [cited 2020 Apr 25]. Available from: https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations
Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104 (3): 246-51.
van Doremalen N., Bushmaker T., Morris D. H., Holbrook M. G., Gamble A., Williamson B. N., Tamin A., Harcourt J. L., Thornburg N. J., Gerber S. I., Lloyd-Smith J. O., de Wit E., Munster V. J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020; 382 (16): 1564-67.
Udugama B., Kadhiresan P., Kozlowski H. N., Malekjahani A., Osborne M., Li V. Y. C., Chen H., Mubareka S., Gubbay J. B., Chan W. C. W. Diagnosing COVID-19: the disease and tools for detection [published online ahead of print, 2020 Mar 30]. ACS Nano. 2020; 14 (4): 3822-35.
Vashist S. K. Point-of-care diagnostics: Recent advances and trends. Biosensors (Basel). 2017; 17 (4): E62.
Cheng M. P., Papenburg J., Desjardins M., Kanjilal S., Quach C., Libman M., Dittrich S., Yansouni C. P. Diagnostic testing for severe acute respiratory syndrome–related coronavirus-2. Ann Intern Med. 2020. doi: 10.7326/M20-1301
Song J., Wang G., Zhang W., Zhang Y., Li W., Zhou Z.; People’s Liberation Army Professional Committee of Critical Care Medicine, Chinese Society on Thrombosis and Haemostasis. Chinese expert consensus on diagnosis and treatment of coagulation dysfunction in. Mil Med Res. 2020; 7 (1): 19.
McCreary E. K., Pogue J. M. Coronavirus disease 2019 treatment: a review of early and emerging options. Open Forum Infect Dis. 2020; 7 (4): ofaa105.
Xiao A. T., Tong Y. X., Gao C., Zhu L., Zhang Y. J., Zhang S. Dynamic profile of RT-PCR findings from 301 COVID-19 patients in Wuhan, China: a descriptive study. J Clin Virol. 2020:104346. doi: 10.1016/j.jcv.2020.104346
Zhai P., Ding Y., Wu X., Long J., Zhong Y., Li Y. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents. 2020: 105955.
Green K., Winter A., Dickinson R., Graziadio S., Wolff R., Mallett S., Allen A. J. What tests could potentially be used for the screening, diagnosis and monitoring of COVID-19 and what are their advantages and disadvantages? Centre for Evidence-Based Medicine. 2020. Available from: https://www.cebm.net/covid-19/what-tests-could-potentially-be-used-for-the-screening-diagnosis-and-monitoring-of-covid-19-and-what-are-their-advantages-and-disadvantages/
Baek Y. H., Um J., Antigua K. J. C., Park J. H., Kim Y., Oh S., Kim Y. I., Choi W. S., Kim S. G., Jeong J. H., Chin B. S., Nicolas H. D. G., Ahn J. Y., Shin K. S., Choi Y. K., Park J. S., Song M. S. Development of a reverse transcription-loop-mediated isothermal amplification as a rapid early-detection method for novel SARS-CoV-2. Emerg Microbes Infect. 2020; 1-31. doi: 10.1080/22221751.2020.1756698
Nguyen T., Duong Bang D., Wolff A. 2019 novel coronavirus disease (COVID-19): paving the road for rapid detection and point-of-care diagnostics. Micromachines (Basel). 2020; 11 (3): 306.
Park G. S., Ku K., Baek S. H., Kim S. J., Kim S. I., Kim B. T., Maeng J. S.Development of reverse transcription loop-mediated isothermal amplification assays targeting SARS-CoV-2. J Mol Diagn. 2020; S1525-1578 (20) 30090-8.
Yan C., Cui J., Huang L., Du B., Chen L., Xue G., Li S., Zhang W., Zhao L., Sun Y., Yao H., Li N., Zhao H., Feng Y., Liu S., Zhang Q., Liu D., Yuan J. Rapid and visual detection of 2019 novel coronavirus (SARS-CoV-2) by a reverse transcription loop-mediated isothermal amplification assay. Clin Microbiol Infect. 2020;S1198-743X(20)30186-5. doi: 10.1016/j.cmi.2020.04.001
Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020. doi: 10.1016/j.jpha.2020.03.001
Sheridan C. Fast, portable tests come online to curb coronavirus pandemic. Nat Biotechnol. 2020. doi: 10.1038/d41587-020-00010-2
Liu R., Fu A., Deng Z., Li Y., Liu T. Promising methods for detection of novel coronavirus SAR-SCoV-2. View. 2020 Mar; 1 (1).
Kakodkar P., Kaka N., Baig M. N. A comprehensive literature review on the clinical presentation, and management of the pandemic coronavirus disease 2019 history of the outbreak. Cureus. 2020; 12 (4): e7560.
Sri Santosh T., Parmar R., Anand H., Srikanth K., Saritha M. A review of salivary diagnostics and its potential implication in detection of Covid-19. Cureus. 2020; 12 (4): e7708.
Azzi L., Carcano G., Gianfagna F., Grossi P., Gasperina D. D., Genoni A., Fasano M., Sessa F., Tettamanti L., Carinci F., Maurino V., Rossi A., Tagliabue A., Baj A. Saliva is a reliable tool to detect SARS-CoV-2. J Infect. 2020: S0163-4453 (20) 30213-9.
Ren Y. F., Rasubala L., Malmstrom H., Eliav E. Dental care and oral health under the clouds of COVID-19. JDR Clin Transl Res. 2020. 238008442092438. doi: 10.1177/2380084420924385
Yousefifard M., Zali A., Ali K. M., Madani Neishaboori A., Zarghi A., Hosseini M., Safari S. Antiviral therapy in management of COVID-19: a systematic review on Current evidence. Arch Acad Emerg Med. 2020; 8 (1): e45.
El-Aziz T. M. A., Stockand J. D. Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2) - an update on the status. Infect Genet Evol. 2020. 104327.
Tobaiqy M., Qashqary M., Al-Dahery S., Mujallad A, Hershan AA, Kamal MA, Helmi N. Therapeutic management of COVID-19 patients: a systematic review. Infect Prev Pract. 2020:100061.
Zhang J., Xie B., Hashimoto K. Current status of potential therapeutic candidates for the COVID-19 crisis. Brain Behav Immun. 2020;S0889-1591 (20) 30589-4.
Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F. G.1, Horby P. W., Zhang D., Wang C. A trial of lopinavir-ritonavir in adults hospitalized with Severe Covid-19 [published online ahead of print, 2020 Mar 18]. N Engl J Med. 2020; NEJMoa2001282.
Meyer S. De, Bojkova D., Cinati J., Damme E. Van, Buyck C., Van Loock M., Woodfall B., Ciesek S. Lack of antiviral activity of darunavir against SARS-CoV-2. medRxiv. doi: 2020.04.03.20052548
Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020. 32253759
Caly L., Druce J. D., Catton M. G., Jans D. A., Wagstaff K. M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro [published online ahead of print, 2020 Apr 3]. Antiviral Res. 2020; 178: 104787.
Marano G., Vaglio S., Pupella S., Facco G., Catalano L., Liumbruno G. M., Grazzini G. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 2016; 14 (2): 152-57.
Ahn J. Y., Sohn Y., Lee S. H., Cho Y., Hyun J. H., Baek Y. J., Jeong S. J., Kim J. H., Ku N. S., Yeom J. S., Roh J., Ahn M. Y., Chin B. S., Kim Y. S., Lee H., Yong D., Kim H. O., Kim S., Choi J. Y. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci. 2020; 35 (14): e149.
Moreno E., Lomonte B., Gutiérrez J. M. Computational biology in Costa Rica: the role of a small country in the global context of bioinformatics. PLoS Comput Biol. 2008; 4 (3): e1000040.
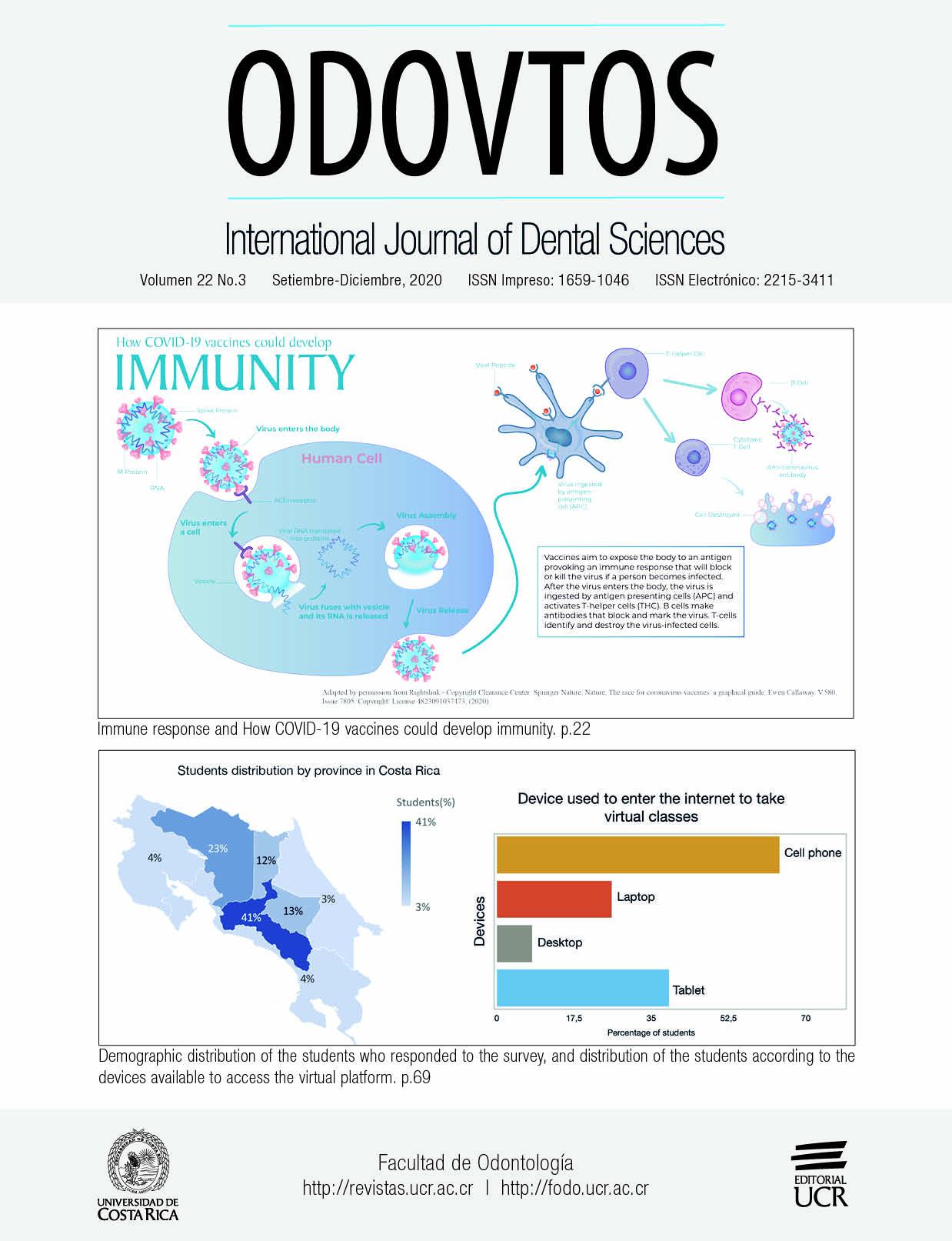
Chart Sources: Nature Analysis Based On Who Covid-19 Vaccine Landscape/Milken Institute Covid-19 Treatment And Vaccine Tracker/T. Thanh Le Et Al. Nature Rev. Drug. Disc. Http://Doi.Org/Ggrnbr (2020)/F. Amanat & F. Krammer Immunity 52, 583-589 (2020)/W. Shang Et Al. Npj Vaccines 5, 18 (2020).