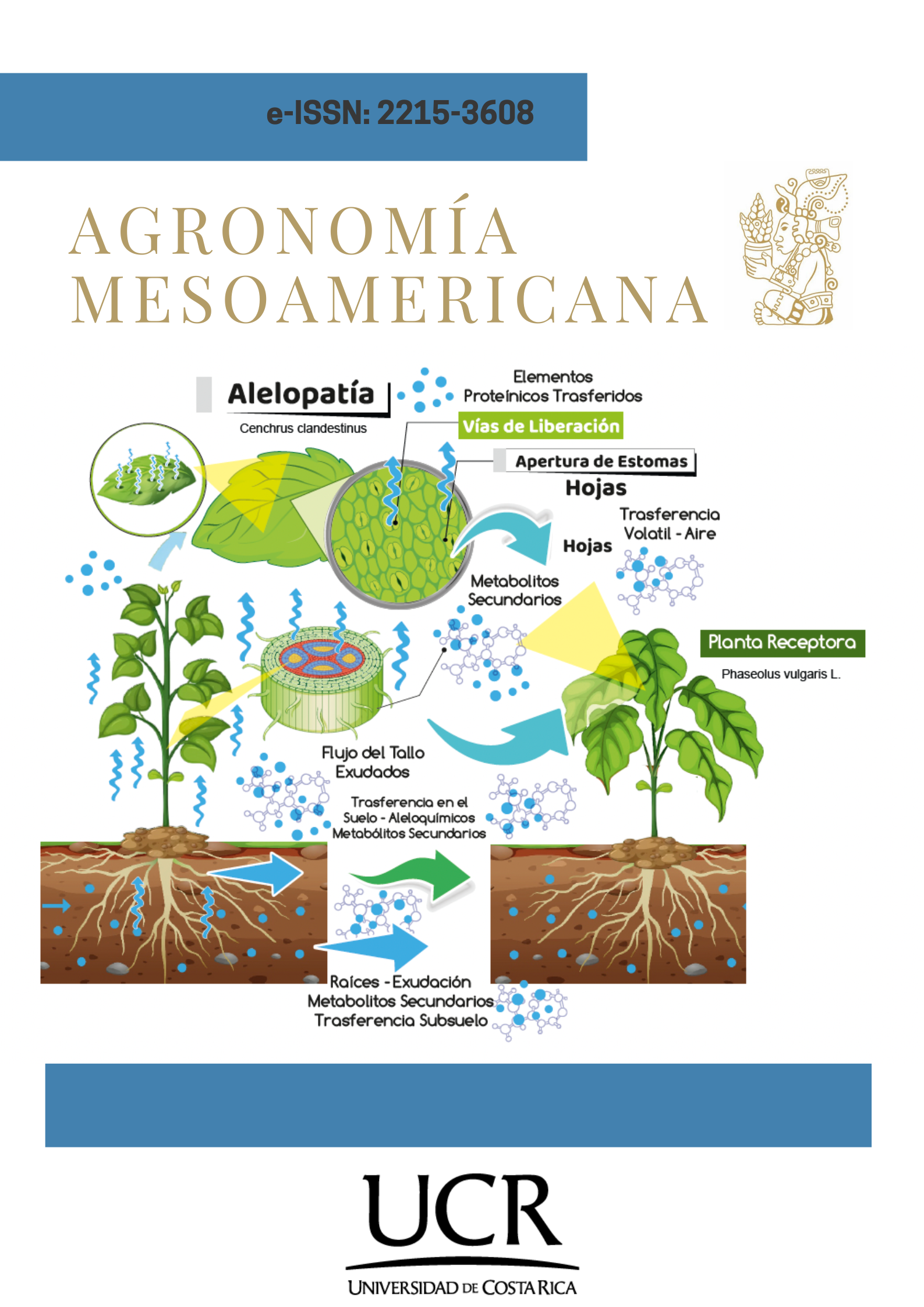
Alelopathy of Cenchrus clandestinus on common bean (Phaseolus vulgaris L.) germination
DOI:
https://doi.org/10.15517/am.2024.54725Keywords:
seeds, bioassays, allelochemicals, extracts, sensitivityAbstract
Introduction. Weeds are one of the main problems in agricultural productivity due to their interactions with crops, such as alelopatic effects on germination. Objective. To evaluate the allelopathy of kikuyo grass (Cenchrus clandestinus) on the germination of beans (Phaseolus vulgaris L.) variety ICA Cerinza. Materials and methods. The study was conducted at the Quality Control Laboratory of the Universidad de Pamplona, Central Campus, Pamplona, Norte de Santander, Colombia, from May to August 2022. To each experimental unit (Petri dishe) with 12 been seeds, 6 mL of kikuyo aqueous extract (leaf, stem, and root) at three concentrations (1, 2.5, and 5 %), plus a control (0 %), were added, totaling 12 treatments. From day 0 to 12, the number of germinated seeds (NGS) was recorded, and the germination rate index (GRI) and germination index (IG) were calculated. The biomolecular profile of the aqueous extract was determined using the Fourier-transform spectroscopy. A completely randomized experimental design with a factorial arrangement (3x4), with repeated measures in each experimental unit, was employed. The intersuject factors were the organ type and concentration, while the intrasubject factor was the evaluation time (12 days). Results. Significant differences (p<0.001) were observed for NSG among treatments due to the concentration of the aqueous extract, as well as for IG, while GRI depended on the organ (p<0.05) and concentration (p<0.001), both negatively affected as the concentration increased. The infrared spectrum of the extract determined the presence of polyphenols, nitriles, and siloxanes. Conclusión. C. clandestinus affected NGS, GRI, and GI of P. vulgaris, with an allelopathic effect of the leaf aqueous extract at higher concentrations. Polyphenols, nitriles, and siloxanes associated with allelopathic effects were identified in the aqueous extracts.
Downloads
References
Abril-Saltos, R. V., Villacis-Estrada, E. A., Tapuy-Andi, M. D., Pillco-Herrera, B. M., Quishpe-Lopez, J. D., & López-Adriano, K. P. (2023). Germinación y crecimiento de Sterculia colombiana en Arosemena Tola, Napo, Ecuador. Agronomía Mesoamericana, 34(2), Artículo 51104. https://doi.org/10.15517/am.v34i2.51104
Abugre, S., Apetorgbor, A. K., Antwiwaa, A., & Apetorgbor, M. M. (2011). Allelopathic effects of ten tree species on germination and growth of four traditional food crops in Ghana. Journal of Agricultural Technology, 7(3), 825–834. https://www.thaiscience.info/journals/Article/IJAT/10842442.pdf
Araniti, F., Mancuso, R., Lupini, A., Giofrè, S. V., Sunseri, F., Gabriele, B., & Abenavoli, M. R. (2015). Phytotoxic potential and biological activity of three synthetic coumarin derivatives as new natural-like herbicides. Molecules, 20(10), 17883–17902. https://doi.org/10.3390/molecules201017883
Arora, K. (2015). Comparative account of allelopathic potential of different parts of Cassia occidentalis and its correlation with bio-molecular profile through FTIR. Journal of Chemical and Pharmaceutical Research, 7(12), 91–95. https://bit.ly/42x45QR
Beć, K. B., Grabska, J, & Huck, C. W. (2020). Biomolecular and bioanalytical applications of infrared spectroscopy – A review. Analytica Chimica Acta, 1133, 150–177. https://doi.org/10.1016/j.aca.2020.04.015
Ben Kaab, S., Lins, L., Hanafi, M., Bettaieb Rebey, I., Deleu, M., Fauconnier, M. -L., Ksouri, R., Haissam Jijakli, M., & De Clerck, C. (2020). Cynara cardunculus crude extract as a powerful natural herbicide and insight into the mode of action of its bioactive molecules. Biomolecules, 10(2), Article 209. https://doi.org/10.3390/biom10020209
Blažević, I., Montaut, S., Burčul, F., Olsen, C. E., Burow, M., Rollin, P., & Agerbirk, N. (2019). Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry, 169, Article 112100. https://doi.org/10.1016/j.phytochem.2019.112100
Brown, P. D., & Morra, M. J. (1995). Glucosinolate-containing plant tissues as bioherbicides. Journal of Agricultural and Food Chemistry, 43, 3070–3074. https://doi.org/10.1021/jf00060a015
Burbano Salas, D. (2019). Uso del Kikuyo (Pennisetum clandestinum L.), residuo de la poda de áreas verdes para la obtención de ácido piroleñoso con fines agropecuarios. Ciencia Digital, 3(3.4.), 354–364. https://doi.org/10.33262/cienciadigital.v3i3.4..884
Cámara de Comercio de Bogotá. (2015). Programa de apoyo agrícola y agroindustrial vicepresidencia de fortalecimiento empresarial cámara de comercio de Bogotá. DOCPLAYER. https://docplayer.es/35498135-Lulo-programa-de-apoyo-agricola-y-agroindustrial-vicepresidencia-de-fortalecimiento-empresarial-camara-de-comercio-de-bogota.html
Carillo, P., Cozzolino, C., D’Abrosca, B., Nacca, F., DellaGreca, M., Fiorentino, A., & Fuggi, A. (2010). Effects of the allelochemicals dihydrodiconiferyl alcohol and lariciresinol on metabolism of Lactuca sativa. The Open Bioactive Compounds Journal, 3, 18–24. https://dx.doi.org/10.2174/1874847301003010018
Castillo-Quiroz, D., Antonio-Bautista, A., Ávila-Flores, D. Y., Sáenz-Reyes, J. T., & Castillo-Reyes, F. (2018). Tratamientos químicos y biológicos para estimular la germinación en semillas de Nolina cespitifera Trel. Polibotánica, 45, 147–156. https://doi.org/10.18387/polibotanica.45.11
Clavijo, J., & Baker, J. B. (1988). Germinacion, emergencia y crecimiento temprano de arroz rojo y cuatro variedades de arroz. Agronomía Colombiana, 5(1-2), 3–7. https://revistas.unal.edu.co/index.php/agrocol/article/view/20931
de Araujo Barbosa, J., Ferreira, S. D., Cologni Salvalaggio, A., Vilanova da Costa, N., & de Moraes Echer, M. (2018). Allelopathy of aqueous Pachyrhizus erosus L. extracts on Euphorbia heterophylla and Bidens pilosa. Pesquisa Agropecuária Tropical, 48, 59–65. https://doi.org/10.1590/1983-40632018v4851117
Federación Nacional de Cultivadores de Cereales, Leguminosas y Soya de Colombia. (2022). Indicadores mensuales. Boletines 2022. https://fenalce.co/indicadores-mensuales/
Flores Córdova, M. A., Sánchez Chávez, E., & Pérez Leal, R. (2015). Potencial Alelopático de extractos foliares de Astragalus mollissimus Torr. sobre la germinación in vitro de semillas de maleza. Revista Mexicana de Ciencias Agrícolas, 6(5), 1093–1103. https://doi.org/10.29312/remexca.v6i5.601
Frisch, T., Motawia, M. S., Olsen, C. E., Agerbirk, N., Møller, B. L., & Bjarnholt, N. (2015). Diversified glucosinolate metabolism: biosynthesis of hydrogen cyanide and of the hydroxynitrile glucoside alliarinoside in relation to sinigrin metabolism in Alliaria petiolata. Frontiers in plant science, 6, Article 926. https://doi.org/10.3389/fpls.2015.00926
Gomes Basso Los, F., Fereira Zielinski, A. A., Wojeicchowski, J. P., Nogueira, A., & Mottin Demiate, I. (2018). Beans (Phaseolus vulgaris L.): whole seeds with complex chemical composition. Current Opinion in Food Science, 19, 63–71. https://doi.org/10.1016/j.cofs.2018.01.010
González-Zertuche, L., & Orozco-Segovia, A. (1996). Métodos de análisis de datos en la germinación de semillas, un ejemplo: Manfreda brachystachya. Boletín de la Sociedad Botánica de México, (58), 15–30. https://doi.org/10.17129/botsci.1484
Hartmann, H. T., & Kester, D. E. (1988). Propagación de plantas y principios básicos (2da ed.). CECSA.
Hasan, M., Ahmad-Hamdani, M. S., Rosli, A. M., & Hamdan, H. (2021). Bioherbicides: An eco-friendly tool for sustainable weed management. Plants, 10(6), Article 1212. https://doi.org/10.3390/plants10061212
Huang, W., Hu, H., Hu, T., Chen, H., Wang, Q., Chen, G., & Tu, L. (2015). Impact of aqueous extracts of Cinnamomum septentrionale leaf litter on the growth and photosynthetic characteristics of Eucalyptus grandis seedlings. New Forests, 46(4), 561–576. https://doi.org/10.1007/s11056-015-9474-8
Hussain, M. I., El-Sheikh, M. A., & Reigosa, M. J. (2020). Allelopathic potential of aqueous extract from Acacia melanoxylon R. Br. on Lactuca sativa. Plants, 9(9), Article 1228. https://doi.org/10.3390/plants9091228
Ibanhes Neto, H. F., Marcato, M. H. F., Marubayashi, R. Y. P., Takahashi, L. S. A., & Dalazen, G. (2020). Germination and initial growth of crops and weeds in response to sourgrass aqueous extracts. Revista de Ciências Agrárias, 43(1), 14–22. https://doi.org/10.19084/rca.18659
International Business Machine. (2015). Software IBM SPSS (version 24). https://www.ibm.com/es-es/spss
Jaimes-Cruz, L. J., Mendoza-Orellana, E., Menjivar-Dominguez, C., Montoya-Almendarez, E., Giraldo-Mejía, Á., & Correa-Cardona, H. (2021). Extrusión húmeda del pasto Kikuyo (Cenchrus clandestinus (Hochst ex Chiov)). Revista MVZ Córdoba, 26(1), Articulo e1964. https://doi.org/10.21897/rmvz.1964
Kumar Patle, T., Shrivas, K., Kurrey, R., Upadhyay, S., Jangde, R., & Chauhan, R. (2020). Phytochemical screening and determination of phenolics and flavonoids in Dillenia pentagyna using UV–vis and FTIR spectroscopy. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 242, Article 118717. https://doi.org/10.1016/j.saa.2020.118717
Labrada, R. (s.f). Recomendaciones para el manejo de malezas. Organización de las Naciones Unidas para la Agricultura y la Alimentación. Recuperado diciembre 7, 2022 de https://www.fao.org/3/a0884s/a0884s.pdf
Laizer, H. C., Chacha, M. N., & Ndakidemi, P. A. (2021). Allelopathic effects of Sphaeranthus suaveolens on seed germination and seedling growth of Phaseolus vulgaris and Oryza sativa. Advances in Agriculture, 2021, Article 8882824. https://doi.org/10.1155/2021/8882824
Lessa, B. F. da T., Silva, M. L. dos S., Barreto, J. H. B., & de Oliveira, A. B. (2017). Efeitos alelopáticos de extratos aquosos de folhas de Amburana cearensis e Plectranthus barbatus na germinação de Amaranthus deflexus. Revista de Ciências Agrárias, 40(1), 79–86. https://doi.org/10.19084/RCA16063
Lv, Q., Li, X., Fan, B., Zhu, C., & Chen, Z. (2022). The Cellular and subcellular organization of the glucosinolate–myrosinase system against herbivores and pathogens. International Journal of Molecular Sciences, 23(3), Article 1577. https://doi.org/10.3390/ijms23031577
Macías, F. A., Mejías, F. J. R., & Molinillo, J. M. G. (2019). Recent advances in allelopathy for weed control: from knowledge to applications. Pest Management Science, 75(9), 2413–2436. https://doi.org/10.1002/ps.5355
Mlombo, N., Dube, Z. P., Ganyani, L., Nxumalo, H., Mnyambo, N. M., & Timana, M. (2021). Argemone ochroleuca extract suppression of germination and early growth of bean (Phaseolus vulgaris). Research on Crops, 22(3), 508–515. https://doi.org/10.31830/2348-7542.2021.098
Mocniak, L. E., Elkin, K., & Bollinger Jr, J. M. (2020). Lifetimes of the aglycone substrates of specifier proteins, the autonomous iron enzymes that dictate the products of the glucosinolate-myrosinase defense system in Brassica plants. Biochemistry, 59(26), 2432–2441. https://doi.org/10.1021/acs.biochem.0c00358
Morales-Santos, M. E., Peña-Valdivia, C. B., García-Esteva, A., Aguilar-Benítez, G., & Kohashi-Shibata, J. (2017). Características físicas y de germinación en semillas y plántulas de frijol (Phaseolus vulgaris L.) silvestre, domesticado y su progenie. Agrociencia, 51, 43–62. https://agrociencia-colpos.org/index.php/agrociencia/article/view/1277/1277
Moreno-Preciado, O. E., & Balaguera-López, H. E. (2021). Caracterización de la comunidad de malezas y su diversidad en una modelación estadística en un cultivo de duraznero (Prunus persica (L.) Batsch.). Revista U.D.C.A Actualidad & Divulgación Científica, 24(1), Article e1734. https://doi.org/10.31910/rudca.v24.n1.2021.1734
Nunes Lopes, R. W., Marques Morais, E., Lacerda, J. J. de J., & da Silva Araújo, F. D. (2022). Bioherbicidal potential of plant species with allelopathic effects on the weed Bidens bipinnata L. Scientific Reports, 12(1), Article 13476. https://doi.org/10.1038/s41598-022-16203-5
Organización de las Naciones Unidas para la Agricultura y la Alimentación. (2006). Procedimientos para el manejo del riesgo de malezas post-entrada. http://www.fao.org/fileadmin/templates/agphome/documents/Biodiversity-pollination/Weeds/Docs/Post-entrada_manejo_Spanish.pdf
Pérez-Peralta, P. J., Ferrera-Cerrato, R., Alarcón, A., Trejo-Téllez, L. I., Cruz-Ortega, R., & Silva-Rojas, H. V. (2019). Respuesta del simbiosistema frijol (Phaseolus vulgaris L.) y Rhizobium tropici CIAT899 ante el efecto alelopático de Ipomoea purpurea L. Roth. Revista Argentina de Microbiología, 51(1), 47–55. https://doi.org/10.1016/j.ram.2018.01.006
Pratibha, G., Rao, K. V., Srinivasa, I., Raju, B. M. K., Shanker, A. K., Madhavi, M., Indoria, A. K., Srinivasa Rao, M., Murthy, K., Sammi Reddy, K., Srinivas Rao, Ch., Biswas, A. K., & Chaudhari, S. K. (2021). Weed shift and community diversity in conservation and conventional agriculture systems in pigeonpea-castor systems under rainfed semi-arid tropics. Soil and Tillage Research, 212, Article 105075. https://doi.org/10.1016/j.still.2021.105075
Putnam, A. R., & Duke, W. B. (1978). Allelopathy in agroecosystems. Annual review of phytopathology, 16, 431–451. https://doi.org/10.1146/annurev.py.16.090178.002243
Quevedo García, E., Arévalo González, M. E., & Cancino Escalante, G. O. (2015). Evaluación de factores abióticos que inciden sobre la germinación de dos biotipos de arroz rojo (Oryza sativa L.). Bistua: Revista de la Facultad de Ciencias Básicas, 13(2), 63–78. https://doi.org/10.24054/01204211.v2.n2.2015.1801
Rigon, J. P., Capuani, S., Cherubin, M. R., Wastowski, A. D., & da Rosa, G. M. (2012). Allelopathic effects of aqueous extract of Brassica napus on germination of seeds of Phaseolus vulgaris. Revista Brasileira de Ciencias Agrárias, 7(3), 451–455. https://doi.org/10.5039/agraria.v7i3a1732
Rodríguez-Gutiérrez, J. L., Correa-Higuera, L. J., Alvarado-Camacho, A. E., & Chaparro-Pesca, J. A. (2016). Evaluación de la actividad alelopática de extractos crudos de Copaifera pubiflora (Benth), sobre la germinación de Mimosa pudica (Lineo). Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales, 40(157), 621–628. https://doi.org/10.18257/raccefyn.379
Santana, D. G., Ranal, M. A., Mustafa, P. C. V., & Silva, R. M. G. (2006). Germination measurements to evaluate allelopathic interactions. Allelopathy Journal, 17(1), 43–52.
Scott, S. J., Jones, R. A., & Williams, W. A. (1984). Review of data analysis methods for seed germination. Crop Science, 24(6), 1192–1199. https://doi.org/10.2135/cropsci1984.0011183X002400060043x
Shankar, S. R. M., Girish, R., Karthik, N., Rajendran, R., & Mahendran, V. S. (2009). Allelopathic effects of phenolics and terpenoids extracted from Gmelina arborea on germination of black gram (Vigna mungo) and green gram (Vigna radiata). Allelopathy Journal, 23(2), 323–332.
Sithara, N. V., Komathi, S., & Rajalakshmi, G. (2017). Identification of bioactive compounds using different solvents through FTIR studies and GCMS analysis. Journal of Medicinal Plants Studies, 5(2), 192–194. https://www.plantsjournal.com/archives/2017/vol5issue2/PartC/5-2-18-454.pdf
Smith, B. C. (2002). Quantitative spectroscopy: Theory and practice (1st ed.). Academic Press.
Soltys, D., Krasuska, U., Bogatek, R., & Gniazdowsk, A. (2013). Allelochemicals as bioherbicides - present and perspectives. In A. J. Price, & J. A. Kelton (Eds.), Herbicides-current research and case studies in use London (pp. 517–542). InTech Publishers.
Taiz, L., Zeiger, E., Møller, I. M., & Murphy, A. (2017). Fisiologia e desenvolvimento vegetal (6ª ed.). Artmed Editora.
Tahseen, Hemanth Kumar, N. K., & Jagannath, S. (2015). Assessment of allelopathic efficacy of Parthenium hysterophorus L. plant parts on seed germination and seedling growth of Phaseolus vulgaris L. Brazilian Journal of Biological Sciences, 2(3), 85–90. http://revista.rebibio.net/v2n3/v02n03a09.pdf
Undabeytia, T., Shuali, U., Nir, S., & Rubin, B. (2021). Applications of chemically modified clay minerals and clays to water purification and slow release formulations of herbicides. Minerals, 11(1), Article 9. https://doi.org/10.3390/min11010009
Vargas Martínez, J. D. J., Sierra Alarcón, A. M., Mancipe Muñoz, E. A., & Avellaneda Avellaneda, Y. (2018). El kikuyo, una gramínea presente en los sistemas de rumiantes en trópico alto colombiano. CES Medicina Veterinaria y Zootecnia, 13(2), 137–156. https://doi.org/10.21615/cesmvz.13.2.4
Downloads
Additional Files
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Sugey Velasco-Villabona, Enrique Quevedo-García, Amanda Lucía Chaparro-García

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
1. Proposed policy for open access journals
Authors who publish in this journal accept the following conditions:
a. Authors retain the copyright and assign to the journal the right to the first publication, with the work registered under the attribution, non-commercial and no-derivative license from Creative Commons, which allows third parties to use what has been published as long as they mention the authorship of the work and upon first publication in this journal, the work may not be used for commercial purposes and the publications may not be used to remix, transform or create another work.
b. Authors may enter into additional independent contractual arrangements for the non-exclusive distribution of the version of the article published in this journal (e.g., including it in an institutional repository or publishing it in a book) provided that they clearly indicate that the work was first published in this journal.
c. Authors are permitted and encouraged to publish their work on the Internet (e.g. on institutional or personal pages) before and during the review and publication process, as it may lead to productive exchanges and faster and wider dissemination of published work (see The Effect of Open Access).