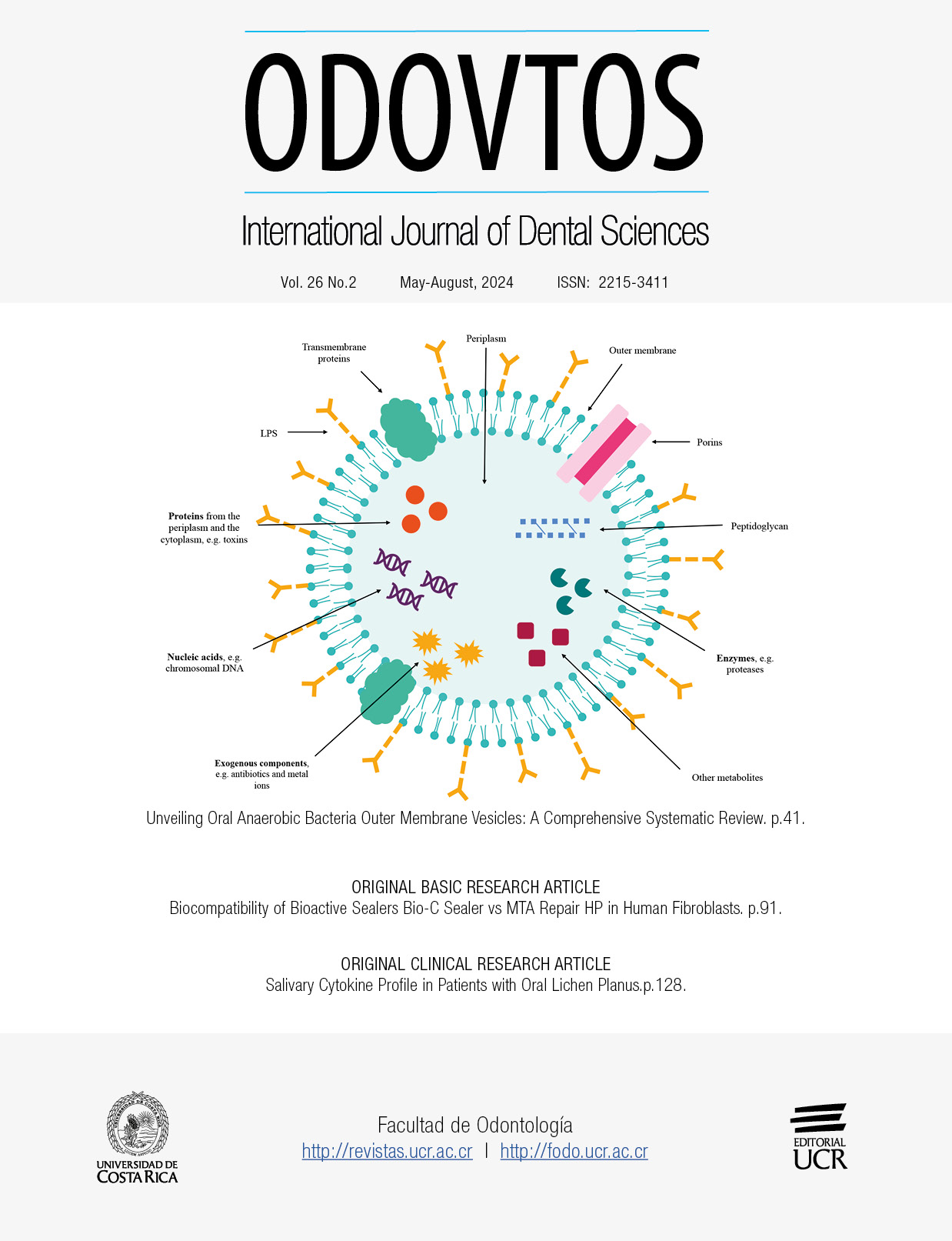
Abstract
Bioactive cements based on tricalcium silicate have been introducedto the market for use in dentistry, with a variety of clinical applications. These cements are in contact with vital tissues such as dental pulp or periodontium in cases of unintentional extrusion; thus, it is important to know the genotoxicity and cytoxicity of these materials. The objective of this study was to evaluate the cytotoxicity and genotoxicity of bioactive sealers, Bio-C® Sealer and MTA Repair HP®, in human fibroblasts. Discs of bioactive sealers Bio-C® Sealer, and MTA Repair HP®, were prepared and set for 24h under sterile conditions. The discs were placed in culture medium at 2.5mg/mL inside a SRT6D roller mixer (Stuart, UK) at 60rpm for 24h. The eluates obtained were incubated for 24h with previously activated and cultured ATCC cell line fibroblasts at 80% confluence. The cytotoxicity was evaluated by Alamar Blue® and LIVE/DEAD assays, as well as the analysis of the Tunel and Mitotracker assays to evaluate genotoxicity using the confocal laser-scanning microscope. In the Alamar Blue® assay, the Bio-C® Sealer presented a cell proliferation of 87%, while the MTA Repair HP® Sealer was 72%. A statistically significant difference was found between the MTA Repair HP® Sealant and the negative control (p=<0.001). Regarding the genotoxicity tests, in the Tunel assay, both materials stain the nucleus of the fibroblast cells exposed to the eluates, while in the Mitotracker assay, the MTA Repair HP® Sealer showed greater mitochondrial function than the Bio-C® Sealer. Calcium silicate-based sealers, Bio-C® Sealer and MTA Repair HP®, are not cytotoxic and have low genotoxicity.
References
Wang Z. Bioceramic materials in endodontics. Endod Topics 2015; 32 (1): 3-30. https://doi.org/10.1111/etp.12075.
Garcez A.S., Ribeiro M.S., Tegos G.P., Núñez S.C., Jorge A.O.C., Hamblin M.R. Antimicrobial photodynamic therapy combined with conventional endodontic treatment to eliminate root canal biofilm infection. Lasers Surg Med 2007; 39 (1): 59-66. doi:10.1002/lsm.20415
Bhat A., Cvach N., Mizuno C., Ahn C., Zhu Q., Primus C., et al. Ion release from prototype surface pre-reacted glass ionomer sealer and EndoSequence BC sealer. Eur Endod J 2021; 6 (1): 122-7. doi:10.14744/eej.2020.50470
Komabayashi T., Colmenar D., Cvach N., Bhat A., Primus C., Imai Y. Comprehensive review of current endodontic sealers. Dent Mater J 2020; 39 (5): 703-20. doi:10.4012/dmj.2019-288
Gasner N.S., Brizuela M. Endodontic materials used to fill root canals. In: StatPearls. StatPearls Publishing 2022.
Orstavik D. Materials used for root canal obturation: technical, biological and clinical testing. Endod Topics 2005; 12 (1): 25-38.
Cintra L.T.A., Benetti F., de Azevedo Queiroz Í.O., de Araújo Lopes J.M., Penha de Oliveira S.H., Sivieri Araújo G., et al. Cytotoxicity, biocompatibility, and biomineralization of the new high-plasticity MTA. J Endod 2017; 43 (5): 774-8. doi:10.1016/j.joen.2016.12.018
Parirokh M., Torabinejad M. Mineral trioxide aggregate: A comprehensive literature rreview-Part III: Clinical applications, drawbacks, and mechanism of action. J Endod 2010; 36 (3): 400-13. doi:10.1016/j.joen.2009.09.009
Prati C., Gandolfi M.G. Calcium silicate bioactive cements: Biological perspectives and clinical applications. Dental Materials 2015; 31 (4): 351-70. doi:10.1016/j.dental.2015.01.004
Zordan-Bronzel C.L., Tanomaru-Filho M., Chávez-Andrade G.M., Torres F.F.E., Abi-Rached G.P.C., Guerreiro-Tanomaru J.M. Calcium Silicate-based experimental sealers: Physicochemical properties evaluation. Mat. Res. 2021; 24 (1). https://doi.org/10.1590/1980-5373-MR-2020-0243
López-García S., Lozano A., Garcíabernal D., Forner L., Llena C., Guerrero-Gironés J., et al. Biological effects of new hydraulic materials on human periodontal ligament stem cells. J Clin Med 2019; 8 (8): 1216. doi:10.3390/jcm8081216
Sultana N., Singh M., Nawal R.R., Chaudhry S., Yadav S., Mohanty S., et al. Evaluation of biocompatibility and osteogenic potential of tricalcium silicate-based cements using human bone marrow-derived mesenchymal stem cells. J Endod 2018; 44 (3): 446-51. doi:10.1016/j.joen.2017.11.016
Alves Silva E.C., Tanomaru-Filho M., da Silva G.F., Delfino M.M., Cerri P.S., Guerreiro-Tanomaru J.M. Biocompatibility and bioactive potential of new calcium silicate-based endodontic sealers: Bio-C sealer and sealer plus BC. J Endod 2020; 46 (10): 1470-7. doi:10.1016/j.joen.2020.07.011
Donnermeyer D., Bürklein S., Dammaschke T., Schäfer E. Endodontic sealers based on calcium silicates: a systematic review. Odontology 2019; 107 (4): 421-36. doi:10.1007/s10266-018-0400-3
Meschi N., Patel B., Ruparel N.B. Material Pulp Cells and Tissue Interactions. J Endod 2020; 46 (9): S150-60. doi:10.1016/j.joen.2020.06.031
Rodríguez-Lozano F.J., López-García S., García-Bernal D., Tomás-Catalá C.J., Santos J.M., Llena C., et al. Chemical composition and bioactivity potential of the new Endosequence BC Sealer formulation HiFlow. Int Endod J 2020; 53 (9): 1216-1228. doi:10.1111/iej.13327
Standarization IO for. ISO 10993-1:2009. In: Biological Evaluation of Medical Devices. 2009.
Okamura T., Chen L., Tsumano S., Ikeda C., Komasa S., Tominaga K., et al. Biocompatibility of a high-plasticity, calcium silicate-based, ready-to-use material. Materials 2020; 13 (21): 4770. doi:10.3390/ma13214770
Bonnier F., Keating M.E., Wróbel T.P., Majzner K., Baranska M., Garcia-Munoz A., et al. Cell viability assessment using the Alamar blue assay: A comparison of 2D and 3D cell culture models. Toxicol in Vitro 2015; 29 (1): 24-131. doi: 10.1016/j.tiv.2014.09.014
Longhin E.M., El Yamani N., Rundén-Pran E., Dusinska M. The alamar blue assay in the context of safety testing of nanomaterials. Front Toxicol 2022; 4. doi:10.3389/ftox.2022.981701
McGaw L.J., Elgorashi E.E., Eloff J.N. Cytotoxicity of African Medicinal Plants Against Normal Animal and Human Cells. In: Toxicological Survey of African Medicinal Plants Elsevier; 2014. p. 181-233.
Klein-Junior C.A., Zimmer R., Dobler T., Oliveira V., Marinowic D.R., Özkömür A., et al. Cytotoxicity assessment of bio-c repair Íon: A new calcium silicate-based cement. J Dent Res Dent Clin Dent Prospects 2021; 15 (3): 152-6. doi:10.34172/joddd.2021.026
Abbaszadeh Z., Çeşmeli S., Biray Avcı Ç. Crucial players in glycolysis: Cancer progress. Gene 2020; 726: 144158. doi:10.1016/j.gene.2019.144158
Guimarães B.M., Prati C., Duarte M.A.H., Bramante C.M., Gandolfi M.G. Physicochemical properties of calcium silicate-based formulations MTA repair HP and MTA vitalcem. Journal of Applied Oral Science 2018; 26. doi:10.1590/1678-7757-2017-0115
Gandolfi M.G., Siboni F., Botero T., Bossù M., Riccitiello F., Prati C. Calcium silicate and calcium hydroxide materials for pulp capping: Biointeractivity, porosity, solubility and bioactivity of current formulations. J Appl Biomater Funct Mater 2015; 13 (1): 43-60. doi:10.5301/jabfm.5000201
Li X., Yoshihara K., De Munck J., Cokic S., Pongprueksa P., Putzeys E., et al. Modified tricalcium silicate cement formulations with added zirconium oxide. Clin Oral Investig 2017; 21 (3): 895-905. doi:10.1007/s00784-016-1843-y
Cardanho-Ramos C., Morais V.A. Mitochondrial biogenesis in neurons: How and where. Int J Mol Sci 2021; 22 (23): 13059. doi:10.3390/ijms222313059
Vergaças J.H.N., de Lima C.O., Barbosa A.F.A., Vieira V.T.L., dos Santos Antunes H., da Silva E.J.N.L. Marginal gaps and voids of three root-end filling materials: A microcomputed tomographic study. Microsc Res Tech 2022; 85 (2): 617-622. doi:10.1002/jemt.23935
Madurantakam P. Which is the superior retrograde filling material? We don’t know! Evid Based Dent 2022; 23 (2): 54-5. doi:10.1038/s41432-022-0237-z
dos Santos Costa F.M., Fernandes M.H., Batistuzzo de Medeiros S.R. Genotoxicity of root canal sealers: a literature review. Clin Oral Investig 2020; 24 (10): 3347-62. doi:10.1007/s00784-020-03478-z
##plugins.facebook.comentarios##

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Copyright (c) 2024 Verónica M. Méndez-González, Joselyn Martínez-López, Amaury Pozos-Guillén, Ana M. González-Amaro, Mariana Gutiérrez-Sánchez, Diana M. Escobar-García