Antifungal activity of Bacillus amyloliquefaciens against Fusarium oxysporum f. sp. cubense race 1
DOI:
https://doi.org/10.15517/am.v32i2.39720Keywords:
antagonist, biological control, Fusarium wilt, metabolitesAbstract
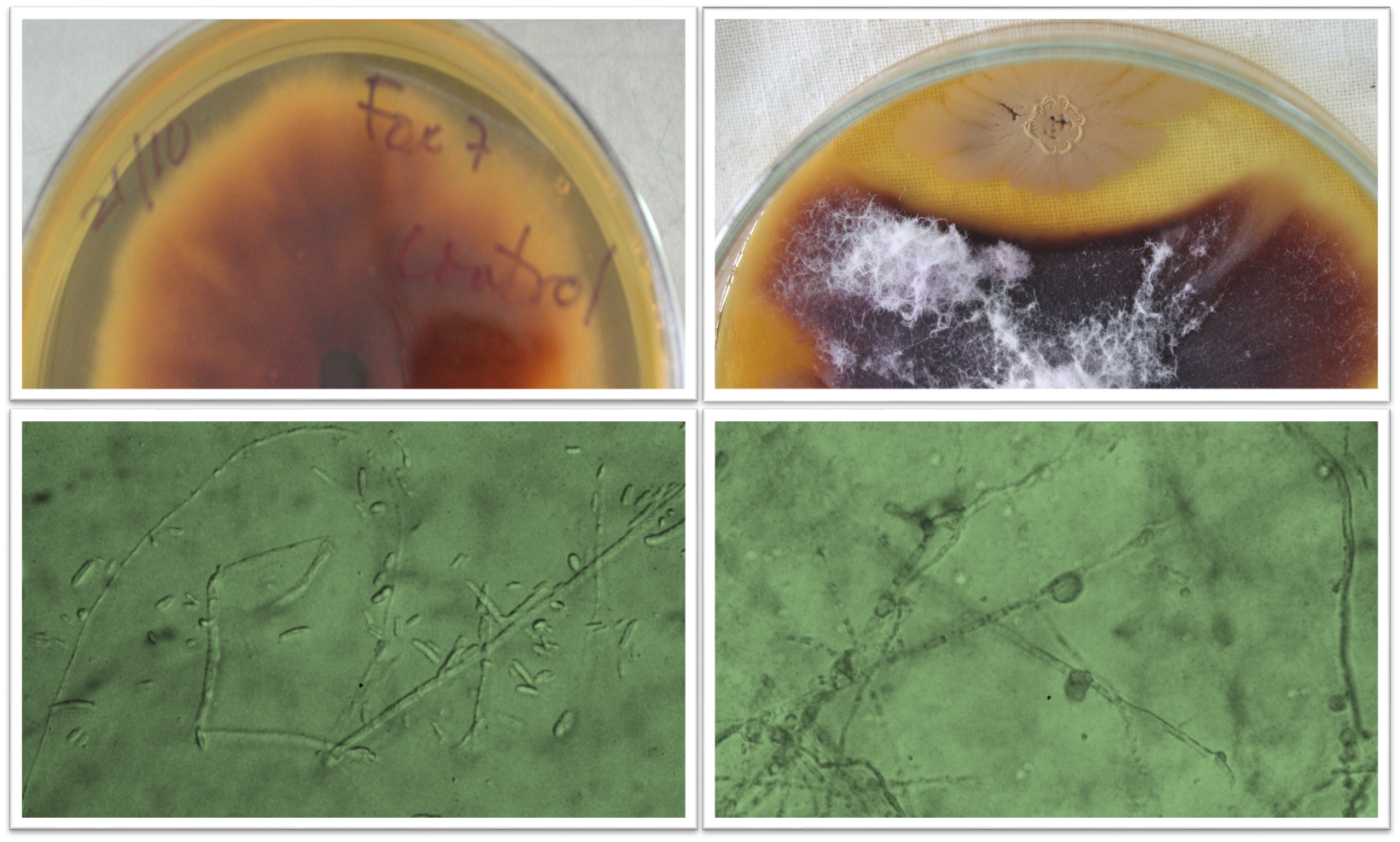
Introduction. Due to the absence of totally effective either economically viable chemical agents for the control of Fusarium wilt, the use of antagonistic microorganisms is of great interest since it could represent a more economically and ecologically sustainable alternative. Objective. To analyze the antifungal effect of the Bacillus amyloliquefaciens CCIBP-A5 strain against Fusarium oxysporum. Materials and methods. The work was carried out in the Laboratory of Applied Microbiology of the Instituto de Biotecnología de las Plantas, Cuba, between September 2017 and June, 2018. The in vitro and in vivo antifungal activity of its culture filtrate and cell against F. oxysporum has been assayed. Results. The results indicated that the metabolites present in the culture filtrate of B. amyloliquefaciens CCIBP-A5 significantly influenced the growth and morphology of the mycelium and the conidia. They also caused oxidative damage to the lipid molecules of F. oxysporum. In addition, this strain showed inhibitory effects on the development of the disease under controlled conditions. These aspects are key when selecting a bacterial candidate as a biological control agent. Conclusions. The results showed that the B. amyloliquefaciens CCIBP-A5 strain, isolated from Musa sp., had an in vitro antifungal effect against the vegetative and reproductive structures of Foc race 1 as well as on the Musa spp.-F. oxysporum interaction. This strain is suggested for the development of a bioproduct for Fusarium wilt management.
Downloads
References
Abdallah, A. B., Jabnoun-Khiareddine, R. H., Mokni-Tlili, S., Nefzi, A., Medimagh-Saidana, S., & Daami-Remadi, M. (2015). Endophytic Bacillus spp. from wild Solanaceae and their antifungal potential against Fusarium oxysporum f. sp. lycopersici elucidated using whole cells, filtrate cultures and organic extracts. Journal of Plant Pathology and Microbiology, 6, 324-330. https://doi.org/10.4172/2157-7471.1000324
Abdallah, A. B., Stedel, C., Garagounis, C., Nefzi, A., Jabnoun-Khiareddine, H., Papadopoulou, K. K., & Daami-Remadi, M. (2017). Involvement of lipopeptide antibiotics and chitinase genes and induction of host defense in suppression of Fusarium wilt by endophytic Bacillus spp. in tomato. Crop Protection, 99, 45-58. https://doi.org/10.1016/j.cropro.2017.05.008
Anthony, K. K., George, D. S., Baldev Singh, H. K., Fung, S. M., Santhirasegaram, V., Razali, Z., & Somasundram, C. (2017). Reactive oxygen species. Activity and antioxidant properties of Fusarium infected bananas. Journal of Phytopathology, 165(4), 213-222. https://doi.org/10.1111/jph.12552
Ayala, A., Muñoz, M., & Argüelles, S. (2014). Lipid Peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-Hydroxy-2Nonenal. Oxidative Medicine and Cellular Longevity, 2014, Article e360438. https://doi.org/10.1155/2014/360438
Bacon, C.W., & Hinton, D.M. (2011). Bacillus mojavensis: its endophytic nature, the surfactins, and their role in the plant response to infection by Fusarium verticillioides. In D.K. Maheshwari (Ed.), Bacteria in agrobiology: Plant growth responses (pp. 21-39). Springer-Verlag.
Broekaert, W.F, Terras, F., Cammue, B., & Vanderleyden, J. (1990). An automated quantitative assay for fungal growth inhibition. FEMS Microbiology Letters, 69, 55-69. https://doi.org/10.1111/j.1574-6968.1990.tb04174.x
Choi, G., Jae Lee, H., & Yun-Cho, K. (1996). Lipid peroxidation and membrane disruption by vinclozolin in dicarboximidesusceptible and resistat isolates of Botrytis cinerea. Pesticide Biochemestry and Physiology, 55(1), 29-39. https://doi.org/10.1006/pest.1996.0032
Chowdhury, S.P., Hartmann, A., Gao, X., & Borriss, R. (2015). Biocontrol mechanism by root-associated Bacillus
amyloliquefaciens FZB42 a review. Frontiers in Microbiology, 6, Article 780. https://doi.org/10.3389/fmicb.2015.00780
Cong, L.L., Sun Y., Wang, J.M., Kang, T.J., Zhang, B., Biligetu, B., & Yang, Q.C. (2017). A rapid screening method for evaluating resistance of alfalfa (Mendicago sativa L.) to Fusarium root rot. Canadian Journal of Plant Pathology, 40, 1-22. https://doi.org/10.1080/07060661.2017.1402822
Cruz-Martín, M., Acosta-Suárez, M., Mena, E., Roque, B., Leiva-Mora, M., Pichardo, T., del Pilar-Castro, R., & Alvarado-Capó, Y. (2013). Cuantificación del crecimiento in vitro de Mycosphaerella fijiensis mediante lecturas de absorbancia. Biotecnología Vegetal, 13(4), 219-224.
Dita-Rodríguez, M., Echegoyen-Ramos, P., & Pérez-Vicente, L. (2013). Plan de contingencia ante un brote de la raza 4 tropical de Fusarium oxysporum f. sp. cubense en un país de la región del OIRSA. Organismo Internacional Regional de Sanidad Agropecuaria. https://www.ippc.int/sites/default/files/documents/20130812/plandecontingenciacontrafocr4toirsa_2013081213%3A52--6.59%20MB.pdf
Dita, M. A., Pérez-Vicente, L., & Martínez, E. (2014). Inoculation of Fusarium oxysporum f. sp. cubense causal agent of Fusarium wilt in banana. In L. F. Pérez-Vicente, M. Dita, E. Martinez de la Parte (Eds.), Technical manual prevention and diagnostic of Fusarium wilt (Panama disease) of banana caused by Fusarium oxysporum f. sp. cubense Tropical Race 4 (TR4) (pp. 55-58). Food and Agriculture Organization.
Fan, H., Zhang, Z., Li, Y., Zhang, X., Duan, Y., & Wang, Q. (2017). Biocontrol of bacterial fruit blotch by Bacillus subtilis 9407 via surfactin-mediated antibacterial activity and colonization. Frontiers in Microbiology, 8, Article 1973. https://doi.org/10.3389/fmicb.2017.01973
Gang, G., Bizun, W., Weihong, M., Xiaofen, L., Xiaolin, Y., Chaohua, Z., Jianhong, M., & Huicai, Z. (2013). Review: Biocontrol of Fusarium wilt of banana: Key influence factors and strategies. African Journal of Microbiology Research, 7, 4835-4843. https://doi.org/10.5897/AJMR2012.2392
Gond, S. K., Marshall, S. B., Torresa, M. S., & White, J. J. (2015). Endophytic Bacillus spp. produce antifungal lipopeptides and induce host defense gene expression in maize. Microbiology Research, 172, 79-87. https://doi.org/.1016/j.micres.2014.11.004
Hernández-Castillo, F., Lira-Saldivar, R.H., Cruz-Chávez, L., Gallegos-Morales, G., Galindo-Cepeda, M., Padrón, E., & Hernández-Suárez, M. (2008). Potencial antifúngico de cepas de Bacillus spp. y extracto de Larrea tridentata contra Rhizoctonia solani en el cultivo de la papa (Solanum tuberosum L.). ÖYTON, 77, 241-252.
Ho, Y. N., Chiang, H. M., Chao, C. P., Su, C. C., Hsu, H. F., Guo, C., Hsieh, J. L., & Huang, C. C. (2015). In planta biocontrol of soilborne Fusarium Wilt of banana through a plant endophytic bacterium, Burkholderia cenocepacia 869T2. Plant Soil, 387, 295-306. https://doi.org/10.1007/s11104-014-2297-0
Huang, Y. H., Wang, R. C., Li, C. H., Zuo, C. W., Wei, Y. R., Zhang, L., & Yi, G. J. (2012). Control of Fusarium wilt in banana with Chinese leek. European Journal of Plant Pathology, 134, 87-95. https://doi.org/10.1007/s10658-012-0024-3
Krieg, N. R., & Holt, J. (1984). Bergey’s manual of systematic bacteriology (9th Ed.) Williams & Wilkins.
Lane, D. (1991). 16S/23S rRNA sequencing. In E. Stackebrandt, & M. Goodfellow (Eds.), Nucleic acid techniques in bacterial systematics (pp. 115-175). John Wiley & Sons, Inc.
Lee, T., Park, D., Kim, K., Lim, S. M., Yu, N. H., Kim, S., Kim, H. Y., Jung, K. S., Jang, J. Y., Park, J. C., Ham, H., Lee, S., Hong, S. K., & Kim, J. C. (2017). Characterization of Bacillus amyloliquefaciens DA12 showing potent antifungal activity against mycotoxigenic Fusarium species. Plant Pathology, 3, 499-507. https://doi.org/10.5423/PPJ.FT.06.2017.0126
Leyva, L., Cruz-Martín, M., Acosta-Suárez, M., Pichardo, T., Bermúdez-Caraballoso, I., & Alvarado-Capó, Y. (2017). In vitro antagonist of Bacillus spp. strains against Fusarium oxysporum f. sp. cubense. Biotecnología Vegetal, 17(4), 229-236.
Li, L., Ma, M., Huang, R., Qu, Q., Li, G., Zhou, J., Zhang, Q., Lu, K., Niu, X., & Luo, J. (2012). Induction of chlamydospore formation in Fusarium by cyclic lipopeptide antibiotics from Bacillus subtilis C2. Journal of Chemistry and Ecology, 38, 966-974. https://doi.org/10.1007/s10886-012-0171-1
Li, L., Qu, Q., Tian B. Y., & Zhang, K. Q. (2005). Induction of chlamydospores in Trichoderma harzianum and Glioladium roseum by antifungal compounds produced by Bacillus subtilis C2. Journal of Phytopathology, 153, 868-693. https://doi.org/10.1111/j.1439-0434.2005.01038.x
Liao, J. H., Chen, P. Y., Yang, Y. L., Kan, S. C., Hsieh, F. C., & Liu, Y. C. (2016). Clarification of the antagonistic effect of the lipopeptides produced by Bacillus amyloliquefaciens BPD1 against Pyricularia oryzae via in situ MALDI-TOF IMS analysis. Molecules, 21, Article 1670. https://doi.org/10.3390/molecules21121670
Liu, K., Newman, M., McInroy, J. A., Hu, C. H., & Kloepper, J. W. (2017). Selection and assessment of plant growth-promoting rhizobacteria for biological control of multiple plant diseases. Phytopathology, 107(8), 928-937. https://dx.doi.org/10.1094/PHYTO-02-17-0051-R
Ng, L. C., Sariah, M., Sariam, O., Radziah, O., & Abidin, M. A. Z. (2016). PGPM-induced defense-related enzymes in aerobic rice against rice leaf blast caused by Pyricularia oryzae. European Journal of Plant Pathology, 145, Article 167. https://dx.doi.org/10.1007/s10658-015-0826-1
Nourozian, J., Etebarian, H. R., & Khodakaramian, G. (2006). Biological control of Fusarium graminearum on wheat by antagonistic bacteria. Songklanakarin Journal of Science and Technology, 28, 29-38.
Pérez-Vicente, L. (2016, April 19-22). Banana farm best practices for prevention of Fusarium wilt TR4 and other exotic banana diseases [Conference presentation]. XXI International ACORBAT Meeting, Miami, FL, USA. https://www.musalit.org/seeMore.php?id=18309
Pérez-Vicente, L., & Dita, M. A. (2014). Fusarium wilt of banana or panama disease by Fusarium oxysporum f. sp. cubense: A review on history, symptoms, biology, epidemiology and management. In L. F. Pérez-Vicente, M. Dita, E. Martinez de la Parte (Eds.), Technical manual prevention and diagnostic of Fusarium wilt (Panama disease) of banana caused by Fusarium oxysporum f. sp. cubense Tropical Race 4 (TR4) (pp. 5-30). Food and Agriculture Organization.
Ploetz, R. C. (2015a). Fusarium wilt of banana. Phytopathology, 105, 1512-1521. http://doi.org/10.1094/PHYTO-04-15-0101-RVW
Ploetz, R. C. (2015b). Management of Fusarium wilt of banana: A review with special reference to tropical race 4. Crop Protection, 73, 7-15. https://doi.org/10.1016/j.cropro.2015.01.007
Radhakrishnan, R., Hashem, A., & Abd-Allah, E.F. (2017). Bacillus: A biological tool for crop improvement through biomolecular changes in adverse environments. Frontiers in Physiology, 8, Article 667. https://doi.org/10.3389/fphys.2017.00667
Raza, W., Ling, N., Yang, L., Huang, Q., & Shen, Q. (2016). Response of tomato wilt pathogen Ralstonia solanacearum to the volatile organic compounds produced by a biocontrol strain Bacillus amyloliquefaciens SQR-9. Scientific Reports, 6, Article 24856. https://doi.org/10.1038/srep24856
Rojas-Solís, D., Contreras-Pérez, M., & Santoyo, G. (2013). Mecanismos de estimulación del crecimiento vegetal en bacterias del género Bacillus. Biológicas, 15(2), 36-41.
Sekhar, A. C., & Thomas, P. (2015). Isolation and identification of shoot-tip associated endophytic bacteria from banana cv. Grand Naine and testing for antagonistic activity against Fusarium oxysporum f. sp. cubense. American Journal of Plant Science, 6, 943-954. https://doi.org/10.4236/ajps.2015.67101
Simonetti, E., Roberts, I. N., Montecchia, M. S., Gutierrez-Boem, F. H., Gomez, F. M., & Ruiz, J. A. (2018). A novel
Burkholderia ambifaria strain able to degrade the mycotoxin fusaric acid and to inhibit Fusarium. Microbiology Research, 206, 50-59. https://doi.org/10.1016/j.micres.2017.09.008
Srivastava, S., Bist, V., Srivastava, S., Singh, P. C., Trivedi, P. K., Asif, M. H., Chauhan, P. S., & Nautiyal, C. S. (2016). Unraveling aspects of Bacillus amyloliquefaciens mediated enhanced production of rice under biotic stress of Rhizoctonia solani. Frontiers in Plant Science, 7, Article 587. https://doi.org/10.3389/fpls.2016.00587
Stover, R. H., & Simmonds N. W. (1987). Bananas (3rd Ed.). Longmans.
Tang, Q., Bie, X., Lu, Z., Lv, F., Tao, Y., & Qu, X. (2014). Effects of fengycin from Bacillus subtilis fmbJ on apoptosis and necrosis in Rhizopus stolonifer. Journal of Microbiology, 2, 675-680. https://doi.org/10.1007/s12275-014-3605-3
Wang, C., Xing, J., Chin, C. K., & Peters, J. S. (2002). Fatty acids with certain structural characteristics are potent inhibitors of germination and inducers of cell death of powdery mildew. Physiology and Molecular Plant Pathology, 61, 151-161. https://doi.org/10.1006/pmpp.2002.0429
Wu, B., Wang, X., Yang, L., Yang, H., Zeng, H., Qiu, Y., & He, Z. (2016). Effects of Bacillus amyloliquefaciens ZM9 on bacterial wilt and rhizosphere microbial communities of tobacco. Applied Soil Ecology, 103, 1-12. https://doi.org/10.1016/j.apsoil.2016.03.002
Wiyono, H., & Widono, S. (2013). Vigor of plantlet from microplantlet treated by filtrate and cell suspension of some isolates of Bacillus and resistance to banana wilt pathogen after acclimatization. ESci Journal of Plant Pathology, 2(2), 70-75.
Xue, C., Penton, C. R., Shen, Z., Zhang, R., Huang, Q., Li, R., Ruan, Y., & Shen, Q. (2015). Manipulating the banana rhizosphere microbiome for biological control of Panama disease. Science Report, 5, Article 11124. https://doi.org/10.1038/srep11124
Yin, H., Xu, L., & Porter, N. (2011). Free radical lipid peroxidation: mechanisms and analysis. Chemical Reviews, 111(10), 5944-5972. https://doi.org/10.1021/cr200084z
Zacky, F. A., & Tiny, S. Y. (2013). Investigating the bioactivity of cells and cell-free extracts of Streptomyces. Biological Control, 66, 204-208. https://doi.org/10.1016/j.biocontrol.2013.06.001
Downloads
Additional Files
Published
How to Cite
Issue
Section
License
1. Proposed policy for open access journals
Authors who publish in this journal accept the following conditions:
a. Authors retain the copyright and assign to the journal the right to the first publication, with the work registered under the attribution, non-commercial and no-derivative license from Creative Commons, which allows third parties to use what has been published as long as they mention the authorship of the work and upon first publication in this journal, the work may not be used for commercial purposes and the publications may not be used to remix, transform or create another work.
b. Authors may enter into additional independent contractual arrangements for the non-exclusive distribution of the version of the article published in this journal (e.g., including it in an institutional repository or publishing it in a book) provided that they clearly indicate that the work was first published in this journal.
c. Authors are permitted and encouraged to publish their work on the Internet (e.g. on institutional or personal pages) before and during the review and publication process, as it may lead to productive exchanges and faster and wider dissemination of published work (see The Effect of Open Access).



























