Optimization of the “Germinator” as a complement for the analysis of seed germination quality in rice (Oryza sativa L.)
DOI:
https://doi.org/10.15517/am.v33iEspecial.50954Keywords:
seed quality, vigor, phenotyping, accelerated aging, controlled agingAbstract
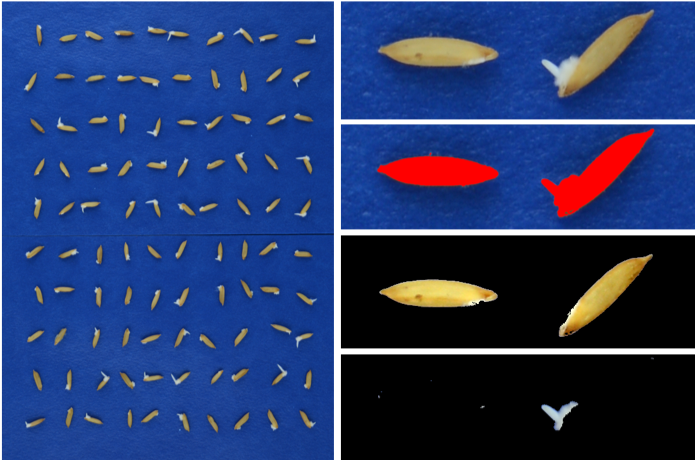
Introduction. The use of digital image analysis with the “Germinator” allows the automatic evaluation of seed germination. In addition to maximum germination, other parameters associated with seed germination vigor can be quantified simultaneously. Objective. To optimize the “Germinator” as a complement for the automatic analysis of seed germination quality in rice. Materials and methods. The experiments were conducted at the Centro para Investigaciones en Granos y Semillas (CIGRAS) from 2015 to 2018. The “Germinator” software package was optimized on the Palmar 18 variety, and then tested with a panel of 126 rice seed samples comprising fourteen varieties. The germination curves were quantified, the automatically acquired data were compared with manual counting based on radical protrusion and a standardized method. Besides, accelerated aging and controlled aging experiments were conducted to show the sensibility of the automatic method. Results. The relationship between germination quantified automatically and quantified manually was high (R2= 0.99). Maximum germination, quantified by image analysis, ranged from 69 % to 100 % in the panel of 126 samples. The correlation between germination assessed by digital images and the standard method was rho (spearman)= 0.34. The “Germinator” allowed the simultaneous quantification of other variables associated with seed vigor, such as the t50 parameter, which is the time the seed lot takes to reach 50 % of the germination. In addition, the automatic method revealed the differential effect of two seed aging protocols. Conclusions. The use of digital image analysis made it possible to evaluate automatically seed germination based on radicle protrusion and also made it possible to quantify other complementary variables associated with seed vigor (t50).
Downloads
References
AL-Tam, F., Adam, H., dos Anjos, A., Lorieux, M., Larmande, P., Ghesquière, A., Jouannic, S., & Shahbazkia, H. R. (2013). P-TRAP: A panicle trait phenotyping tool. BMC Plant Biology, 13(1), Article 122. https://doi.org/10.1186/1471-2229-13-122
Alvarez, J., Carbonell, V., Martinez, E., & Florez, M. (2019). The use of Peleg’s equation to model water absorption in triticale (x Triticosecale wittmack) seeds magnetically treated before soaking. Romanian Journal of Physics, 64, Article 810. https://rjp.nipne.ro/2019_64_3-4/RomJPhys.64.810.pdf
Carpenter, A. E., Jones, T. R., Lamprecht, M. R., Clarke, C., Kang, I. H., Friman, O., Guertin, D. A., Chang, J. H., Lindquist, R. A., Moffat, J., Golland, P., & Sabatini, D. M. (2006). CellProfiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biology, 7(10), Article R100. https://doi.org/10.1186/gb-2006-7-10-r100
Colmer, J., O’Neill, C. M., Wells, R., Bostrom, A., Reynolds, D., Websdale, D., Shiralagi, G., Lu, W., Lou, Q., Le Cornu, T., Ball, J., Renema, J., Flores Andaluz, G., Benjamins, R., Penfield, S., & Zhou, J. (2020). SeedGerm: A cost-effective phenotyping platform for automated seed imaging and machine-learning based phenotypic analysis of crop seed germination. New Phytologist, 228(2), 778–793. https://doi.org/10.1111/nph.16736
Dell’Aquila, A. (2004). Application of a computer-aided image analysis system to evaluate seed germination under different environmental conditions. Italian Journal of Agronomy, 8(1), 51–62. https://bit.ly/3RNmfrS
El-Kassaby, Y. A., Moss, I., Kolotelo, D., & Stoehr, M. (2008). Seed germination: mathematical representation and parameters extraction. Forest Science, 54(2), 220–227. https://doi.org/10.1093/forestscience/54.2.220
Ellis, R. H., & Roberts, E. H. (1980). Improved equations for the prediction of seed longevity. Annals of Botany, 45(1), 13–30. https://doi.org/10.1093/oxfordjournals.aob.a085797
ElMasry, G., ElGamal, R., Mandour, N., Gou, P., Al-Rejaie, S., Belin, E., & Rousseau, D. (2020). Emerging thermal imaging techniques for seed quality evaluation: Principles and applications. Food Research International, 131, Article 109025. https://doi.org/10.1016/j.foodres.2020.109025
Gehan, M. A., Fahlgren, N., Abbasi, A., Berry, J. C., Callen, S. T., Chavez, L., Doust, A. N., Feldman, M. J., Gilbert, K. B., Hodge, J. G., Hoyer, J. S., Lin, A., Liu, S., Lizárraga, C., Lorence, A., Miller, M., Platon, E., Tessman, M., & Sax, T. (2017). PlantCV v2: Image analysis software for high-throughput plant phenotyping. PeerJ, 5, Article e4088. https://doi.org/10.7717/peerj.4088
Hay, F. R., Adams, J., Manger, K., & Probert, R. (2008). The use of non-saturated lithium chloride solutions for experimental control of seed water content. Seed Science and Technology, 36(3), 737–746. https://doi.org/10.15258/sst.2008.36.3.23
International Seed Testing Association. (2018). International rules for seed testing. In International Seed Testing Association (Ed.), Seed vigour testing (2018 ed., pp. i15-1-i15-16). International Seed Testing Association.
International Seed Testing Association. (2022). International rules for seed testing. In International Seed Testing Association (Eds.), The germination test (2022 ed., pp. i5-1-i5.53). International Seed Testing Association.
Jimenez, J. A., Coyne, D. P., Anderson, F. N., & Pavlish, L. A. (1989). Imbibition of seed of dry bean cultivars stored under high or low temperature and relative humidity conditions. Scientia Horticulturae, 40(2), 91–98. https://doi.org/10.1016/0304-4238(89)90090-3
Joosen, R. V. L., Kodde, J., Willems, L. A. J., Ligterink, W., van der Plas, L. H. W., & Hilhorst, H. W. M. (2010). Germinator: A software package for high-throughput scoring and curve fitting of Arabidopsis seed germination. The Plant Journal, 62(1), 148–159. https://doi.org/10.1111/j.1365-313X.2009.04116.x
Kim, J. H., Kim, K. O., Lee, A. K., Roh, M. S., & Suh, J. K. (2017). Germination of Corylopsis seeds evaluated by X-ray imaging and cold stratification. Horticultural Science, 44(2), 105–111. https://doi.org/10.17221/194/2015-HORTSCI
Lurstwut, B., & Pornpanomchai, C. (2017). Image analysis based on color, shape and texture for rice seed (Oryza sativa L.) germination evaluation. Agriculture and Natural Resources, 51(5), 383–389. https://doi.org/10.1016/j.anres.2017.12.002
Moreno-Pizani, M. A., Farias-Ramirez, A. J., Thaner dos Santos,H., da Luz Coelho Novembre, A. D., Guevara-Orozco, L. I., Paredes-Trejo, F., Marin, F. R., da Silva Dias, N., & Alves Marques, P. A. (2019). Qualitative and quantitative evaluation protocol of Baccharis seed germination. Journal of Agricultural Science, 11(3), 421-434. https://doi.org/10.5539/jas.v11n3p421
Mussadiq, Z., Laszlo, B., Helyes, L., & Gyuricza, C. (2015). Evaluation and comparison of open source program solutions for automatic seed counting on digital images. Computers and Electronics in Agriculture, 117, 194–199. https://doi.org/10.1016/j.compag.2015.08.010
Newton, R., Hay, F., & Probert, R. (2009). Protocol for comparative seed longevity testing. [Technical Information Sheet 01]. Millenniun Seed Bank Project Kew. https://doi.org/10.13140/RG.2.2.23886.25921
Nguyen, G. N., & Norton, S. L. (2020). Genebank phenomics: A strategic approach to enhance value and utilization of crop germplasm. Plants, 9(7), Article 817. https://doi.org/10.3390/plants9070817
Oficina Nacional de Semillas. (2019). Estadísticas Comercialización y Producción de Semilla de Arroz. Programa de certificación de semilla de arroz. https://bit.ly/3U5HWoi
Oficina Nacional de Semillas. (2020). Características varietales de arroz (julio, 2020). Programa de certificación de semilla de arroz. https://bit.ly/3U4W2qa
Olesen, M. H., Duijn, B. van, & Boelt, B. (2014). Seed Testing International. In International Seed Testing Association (Eds.), Introduction of new methods: Spectral imaging (ISTA News Bulletin No. 147; pp.10-13) International Seed Testing Association.
Onwimol, D., Chanmprasert, W., Changsee, P., & Rongsangchaichareon, T. (2016). Seed vigor classification using analysis of mean radicle emergence time and single counts of radicle emergence in rice (Oryza sativa L.) and mung bean (Vigna radiata (L.) Wilczek). Agriculture and Natural Resources, 50(5), 345–350. https://doi.org/10.1016/j.anres.2016.12.003
Rajjou, L., Duval, M., Gallardo, K., Catusse, J., Bally, J., Job, C., & Job, D. (2012). Seed germination and vigor. Annual Review of Plant Biology, 63, 507–533. https://doi.org/10.1146/annurev-arplant-042811-105550
Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., Tinevez, J. -Y., White, D. J., Hartenstein, V., Eliceiri, K., Tomancak, P., & Cardona, A. (2012). Fiji: An open-source platform for biological-image analysis. Nature Methods, 9(7), 676-682. https://doi.org/10.1038/nmeth.2019
Severini, A. D., Borrás, L., & Cirilo, A. G. (2011). Counting maize kernels through digital image analysis. Crop Science, 51(6), 2796–2800. https://doi.org/10.2135/cropsci2011.03.0147
Sun, Q., Wang, J., & Sun, B. (2007). Advances on seed vigor physiological and genetic mechanisms. Agricultural Sciences in China, 6(9), 1060–1066. https://doi.org/10.1016/S1671-2927(07)60147-3
Tanabata, T., Shibaya, T., Hori, K., Ebana, K., & Yano, M. (2012). SmartGrain: High-throughput phenotyping software for measuring seed shape through image analysis. Plant Physiology, 160(4), 1871–1880. https://doi.org/10.1104/pp.112.205120
Vidigal, D. S., Marques, A. C. S. S., Willems, L. A. J., Buijs, G., Méndez-Vigo, B., Hilhorst, H. W. M., Bentsink, L., Picó, F. X., & Alonso-Blanco, C. (2016). Altitudinal and climatic associations of seed dormancy and flowering traits evidence adaptation of annual life cycle timing in Arabidopsis thaliana. Plant, Cell & Environment, 39(8), 1737–1748. https://doi.org/10.1111/pce.12734
Wagner, M., Demilly, D., Ducournau, S., Dürr, C., & Léchappé, J. (2011). Computer vision for monitoring seed germination from dry state to young seedlings. Seed Test, 142, 49–51. https://www.seedtest.org/api/rm/G8F2GEQMB2N8K53/sti14249-51.pdf
Walters, C. (1998). Understanding the mechanisms and kinetics of seed aging. Seed Science Research, 8(2), 223–244. https://doi.org/10.1017/S096025850000413X
Wang, P., Fu, N., Li, D., & Wang, L. (2017). Predicting storage conditions for rice seed with thermodynamic analysis. International Journal of Food Engineering, 13(11), Article 20170129. https://doi.org/10.1515/ijfe-2017-0129
Watt, M., Fiorani, F., Usadel, B., Rascher, U., Muller, O., & Schurr, U. (2020). Phenotyping: New windows into the plant for breeders. Annual Review of Plant Biology, 71, 689–712. https://doi.org/10.1146/annurev-arplant-042916-041124
Whitehouse, K. J., Hay, F. R., & Ellis, R. H. (2015). Increases in the longevity of desiccation-phase developing rice seeds: Response to high-temperature drying depends on harvest moisture content. Annals of Botany, 116(2), 247–259. https://doi.org/10.1093/aob/mcv091
Wijesinghe, R. E. H., Lee, S. -Y., Kim, P., Jung, H. -Y., Jeon, M., & Kim, J. (2017). Optical sensing method to analyze germination rate of Capsicum annum seeds treated with growth-promoting chemical compounds using optical coherence tomography. Journal of Biomedical Optics, 22(9), Article 091502. https://doi.org/10.1117/1.JBO.22.9.091502
Yin, G., Whelan, J., Wu, S., Zhou, J., Chen, B., Chen, X., Zhang, J., He, J., Xin, X., & Lu, X. (2016). Comprehensive mitochondrial metabolic shift during the critical node of seed ageing in rice. PLOS ONE, 11(4), Article e0148013. https://doi.org/10.1371/journal.pone.0148013
Zerpa-Catanho, D., Hernández-Pridybailo, A., Madrigal-Ortiz, V., Zúñiga-Centeno, A., Porras-Martínez, C., Jiménez, V. M., & Barboza-Barquero, L. (2019). Seed germination of pitaya (Hylocereus spp.) as affected by seed extraction method, storage, germination conditions, germination assessment approach and water potential. Journal of Crop Improvement, 33(3), 372–394. https://doi.org/10.1080/15427528.2019.1604457
Downloads
Additional Files
Published
How to Cite
Issue
Section
License
1. Proposed policy for open access journals
Authors who publish in this journal accept the following conditions:
a. Authors retain the copyright and assign to the journal the right to the first publication, with the work registered under the attribution, non-commercial and no-derivative license from Creative Commons, which allows third parties to use what has been published as long as they mention the authorship of the work and upon first publication in this journal, the work may not be used for commercial purposes and the publications may not be used to remix, transform or create another work.
b. Authors may enter into additional independent contractual arrangements for the non-exclusive distribution of the version of the article published in this journal (e.g., including it in an institutional repository or publishing it in a book) provided that they clearly indicate that the work was first published in this journal.
c. Authors are permitted and encouraged to publish their work on the Internet (e.g. on institutional or personal pages) before and during the review and publication process, as it may lead to productive exchanges and faster and wider dissemination of published work (see The Effect of Open Access).



























