Abstract
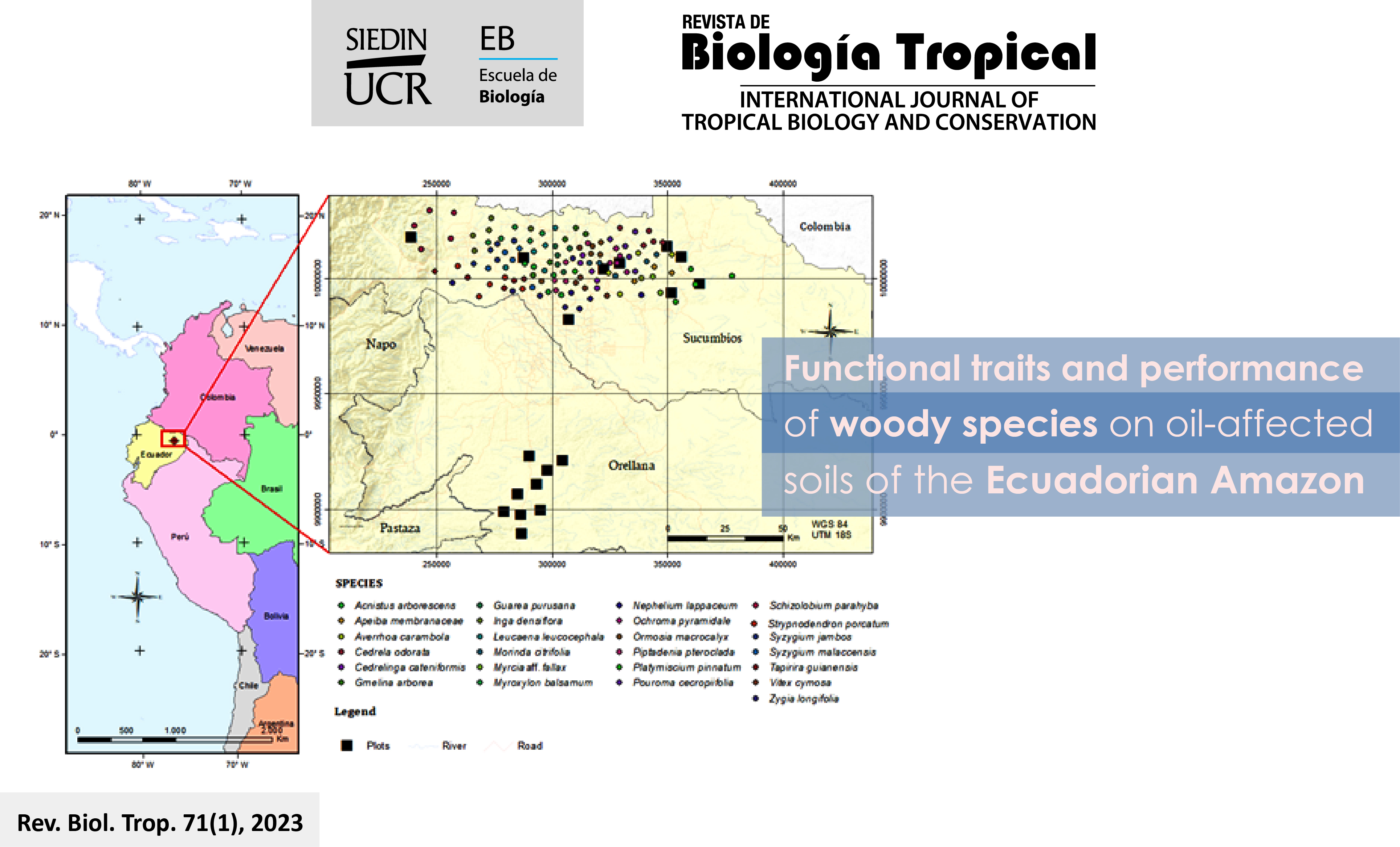
Introduction: Plant functional traits are widely used to predict community productivity. However, they are rarely used to predict the performance (in terms of growth diameter, growth height, survival, and integral response index) of woody species planted in degraded soils. Objective: To evaluate the relationship between the functional traits and the performance of 25 woody species planted in disturbed soils affected by oil extraction activities in Ecuadorian Amazon. Methods: Eighteen permanent sampling plots were established and five 6-month-old seedlings of each 25 species were randomly planted in each plot (125 individuals per plot), at a distance of 4×4 m. Eight quantitative functional traits (leaf size, specific leaf area, leaf nitrogen concentration, leaf phosphorus concentration, leaf minimum unit, leaf dry matter content, stem specific density and leaf tensile strength) were determined for each species. Results: The woody species with high performance shows greater leaf size, specific leaf area and Stem Specific Density than those showing low performance. Leaf nitrogen concentration and stem specific density had a direct relationship with the integral response index. The leaf size, leaf phosphorus concentration, leaf dry matter content and leaf tensile strength sows a negative relationship with the integral response index. Conclusions: Our study showed that the performance of woody species o disturbed soils can be predicted satisfyingly by leaf and stem functional traits, presumably because these traits capture most of environmental and neighborhood conditions.
References
Adler, P. B., Salguero-Gómez, R., Compagnoni, A., Hsu, J. S., Ray-Mukherjee, J., Mbeau-Ache, C., & Franco, M. (2014). Erratum: Functional traits explain variation in plant life history strategies(Proc Natl Acad Sci USA (2014) 111, 2 (740-745) DOI:10.1073/pnas.1315179111). Proceedings of the National Academy of Sciences of the United States of America, 111(27), 10019. https://doi.org/10.1073/pnas.1410430111
Arroyo-Rodríguez, V., Mandujano, S., Benítez-Malvido, J., & Cuende-Fanton, C. (2007). The influence of large tree density on howler monkey (Alouatta palliata mexicana) presence in very small rain forest fragments. Biotropica, 39(6), 760–766. https://doi.org/10.1111/j.1744-7429.2007.00330.x
Bai, X. Y., Wang, S. J., & Xiong, K. N. (2013). Assessing Spatial-Temporal Evolution Processes Of Karst Rocky Desertification Land: Indications For Restoration Strategies. Land Degradation and Development, 24(1), 47–56. https://doi.org/10.1002/ldr.1102
Bertzky, M., Ravilious, C., Kapos, V., Dickson, B., Carrión, D., & Chiu, M. (2011). Carbono, biodiversidad y servicios ecosistémicos: explorando los beneficios múltiples. Unep-Wcmc.
Blossey, B., & Notzold, R. (1995). Evolution of Increased Competitive Ability in Invasive Nonindigenous Plants: A Hypothesis. The Journal of Ecology, 83(5), 887. https://doi.org/10.2307/2261425
Bongers, F., & Popma, J. (1990). OF THE TROPICAL LEAF CHARACTERISTICS. 151(3), 354–365.
Bunn, W. A., Jenkins, M. A., Brown, C. B., & Sanders, N. J. (2010). Change within and among forest communities: The influence of historic disturbance, environmental gradients, and community attributes. Ecography, 33(3), 425–434. https://doi.org/10.1111/j.1600-0587.2009.06016.x
Cernusak, L. A., Winter, K., & Turner, B. L. (2010). Leaf nitrogen to phosphorus ratios of tropical trees: Experimental assessment of physiological and environmental controls. New Phytologist, 185(3), 770–779. https://doi.org/10.1111/j.1469-8137.2009.03106.x
Chaturvedi, R. K., Raghubanshi, A. S., & Singh, J. S. (2011). Leaf attributes and tree growth in a tropical dry forest. Journal of Vegetation Science, 22(5), 917–931. https://doi.org/10.1111/j.1654-1103.2011.01299.x
Chave, J., & Sabatier, U. P. (2006). Measuring wood density for tropical forest trees a field manual. http://www.rainfor.org/upload/ManualsEnglish/wood_density_english[1].pdf
Chigani, H. K., Khajeddin, S. J., & Karimzadeh, H. R. (2012). Soil-vegetation relationships of three arid land plant species and their use in rehabilitating degraded sites. Land Degradation and Development, 23(1), 92–101. https://doi.org/10.1002/ldr.1057
Cornelissen, J. H. C., Lavorel, S., Garnier, E., Díaz, S., Buchmann, N., Gurvich, D. E., Reich, P. B., Ter Steege, H., Morgan, H. D., Van Der Heijden, M. G. A., Pausas, J. G., & Poorter, H. (2003). A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany, 51(4), 335–380. https://doi.org/10.1071/BT02124
Denslow, J. S. (1996). Functional Group Diversity and Responses to Disturbance. 122, 127–151. https://doi.org/10.1007/978-3-642-79755-2_7
Di Rienzo, J., F. Casanoves, M. Balzarini, L. González, M. T. & C. R. (2018). No Title.
Díaz, S., Hodgson, J. G., Thompson, K., Cabido, M., Cornelissen, J. H. C., Jalili, A., Montserrat-Martí, G., Grime, J. P., Zarrinkamar, F., Asri, Y., Band, S. R., Basconcelo, S., Castro-Díez, P., Funes, G., Hamzehee, B., Khoshnevi, M., Pérez-Harguindeguy, N., Pérez-Rontomé, M. C., Shirvany, A., … Zak, M. R. (2004). The plant traits that drive ecosystems: Evidence from three continents. Journal of Vegetation Science, 15(3), 295. https://doi.org/10.1658/1100-9233(2004)015[0295:tpttde]2.0.co;2
Field, C. & H. M. (1986). The photosynthesis nitrogen relationship in wild plants.
Flynn, D. F. B., Gogol-Prokurat, M., Nogeire, T., Molinari, N., Richers, B. T., Lin, B. B., Simpson, N., Mayfield, M. M., & DeClerck, F. (2009). Loss of functional diversity under land use intensification across multiple taxa. Ecology Letters, 12(1), 22–33. https://doi.org/10.1111/j.1461-0248.2008.01255.x
Fortunel, C., Valencia, R., Wright, S. J., Garwood, N. C., & Kraft, N. J. B. (2016). Functional trait differences influence neighbourhood interactions in a hyperdiverse Amazonian forest. Ecology Letters, 19(9), 1062–1070. https://doi.org/10.1111/ele.12642
Freschet, G. T., Cornelissen, J. H. C., van Logtestijn, R. S. P., & Aerts, R. (2010). Substantial nutrient resorption from leaves, stems and roots in a subarctic flora: What is the link with other resource economics traits? New Phytologist, 186(4), 879–889. https://doi.org/10.1111/j.1469-8137.2010.03228.x
Gibert, A., Gray, E. F., Westoby, M., Wright, I. J., & Falster, D. S. (2016). On the link between functional traits and growth rate: meta-analysis shows effects change with plant size, as predicted. Journal of Ecology, 104(5), 1488–1503. https://doi.org/10.1111/1365-2745.12594
Grime, J. P., & Hunt, R. (1975). Relative Growth-Rate: Its Range and Adaptive Significance in a Local Flora. The Journal of Ecology, 63(2), 393. https://doi.org/10.2307/2258728
Guaranda, A. (2016). Apuntes sobre la explotación petrolera en el Ecuador.
Hacke, U. G., Sperry, J. S., Pockman, W. T., Davis, S. D., & McCulloh, K. A. (2001). Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia, 126(4), 457–461. https://doi.org/10.1007/s004420100628
Han, W., Fang, J., Guo, D., & Zhang, Y. (2005). Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytologist, 168(2), 377–385. https://doi.org/10.1111/j.1469-8137.2005.01530.x
Hatfield, J. H., Harrison, M. L. K., & Banks-Leite, C. (2018). Functional Diversity Metrics: How They Are Affected by Landscape Change and How They Represent Ecosystem Functioning in the Tropics. Current Landscape Ecology Reports, 3(2), 35–42. https://doi.org/10.1007/s40823-018-0032-x
Iida, Y., Kohyama, T. S., Swenson, N. G., Su, S. H., Chen, C. T., Chiang, J. M., & Sun, I. F. (2014). Linking functional traits and demographic rates in a subtropical tree community: The importance of size dependency. Journal of Ecology, 102(3), 641–650. https://doi.org/10.1111/1365-2745.12221
King, D. A., Davies, S. J., Nur Supardi, M. N., & Tan, S. (2005). Tree growth is related to light interception and wood density in two mixed dipterocarp forests of Malaysia. Functional Ecology, 19(3), 445–453. https://doi.org/10.1111/j.1365-2435.2005.00982.x
Lake, J. C., & Leishman, M. R. (2004). Invasion success of exotic plants in natural ecosystems: The role of disturbance, plant attributes and freedom from herbivores. Biological Conservation, 117(2), 215–226. https://doi.org/10.1016/S0006-3207(03)00294-5
Le Roux, X., Walcroft, A. S., Daudet, F. A., Sinoquet, H., Chaves, M. M., Rodrigues, A., & Osorio, L. (2001). Photosynthetic light acclimation in peach leaves: Importance of changes in mass:area ratio, nitrogen concentration, and leaf nitrogen partitioning. Tree Physiology, 21(6), 377–386. https://doi.org/10.1093/treephys/21.6.377
Lewis, S. L., Lloyd, J., Sitch, S., Mitchard, E. T. A., & Laurance, W. F. (2009). Changing ecology of tropical forests: Evidence and drivers. Annual Review of Ecology, Evolution, and Systematics, 40, 529–549. https://doi.org/10.1146/annurev.ecolsys.39.110707.173345
Lusk, C. H. (2002). Leaf area accumulation helps juvenile evergreen trees tolerate shade in a temperate rainforest. Oecologia, 132(2), 188–196. https://doi.org/10.1007/s00442-002-0974-9
Maire, V., Gross, N., Hill, D., Martin, R., Wirth, C., Wright, I. J., & Soussana, J. F. (2013). Disentangling Coordination among Functional Traits Using an Individual-Centred Model: Impact on Plant Performance at Intra- and Inter-Specific Levels. PLoS ONE, 8(10), 1–16. https://doi.org/10.1371/journal.pone.0077372
Malhi, Y. (2010). The carbon balance of tropical forest regions, 1990-2005. Current Opinion in Environmental Sustainability, 2(4), 237–244. https://doi.org/10.1016/j.cosust.2010.08.002
Marks, C. O., & Lechowicz, M. J. (2006). Alternative designs and the evolution of functional diversity. American Naturalist, 167(1), 55–66. https://doi.org/10.1086/498276
Martin, P. H., Fahey, T. J., & Sherman, R. E. (2011). Vegetation zonation in a neotropical montane forest: Environment, disturbance and ecotones. Biotropica, 43(5), 533–543. https://doi.org/10.1111/j.1744-7429.2010.00735.x
Martínez-Encino, C., Villanueva-López, G., & Casanova-Lugo, F. (2013). Densidad y composición de árboles dispersos en potreros en la Sierra de Tabasco, México. Agrociencia, 47(5), 483–496.
Martínez-Garza, C., Bongers, F., & Poorter, L. (2013). Are functional traits good predictors of species performance in restoration plantings in tropical abandoned pastures? Forest Ecology and Management, 303(November 2018), 35–45. https://doi.org/10.1016/j.foreco.2013.03.046
Mayaux, P., Holmgren, P., Achard, F., Eva, H., Stibig, H. J., & Branthomme, A. (2005). Tropical forest cover change in the 1990s and options for future monitoring. Philosophical Transactions of the Royal Society B: Biological Sciences, 360(1454), 373–384. https://doi.org/10.1098/rstb.2004.1590
Messier, J., McGill, B. J., & Lechowicz, M. J. (2010). How do traits vary across ecological scales? A case for trait-based ecology. Ecology Letters, 13(7), 838–848. https://doi.org/10.1111/j.1461-0248.2010.01476.x
Mooney, H. A., Field, C., Gulmon, S. L., & Bazzaz, F. A. (1981). Photosynthetic capacity in relation to leaf position in desert versus old-field annuals. Oecologia, 50(1), 109–112. https://doi.org/10.1007/BF00378802
Muller-Landau, H. C. (2004). Interspecific and inter-site variation in wood specific gravity of tropical trees. Biotropica, 36(1), 20–32. https://doi.org/10.1111/j.1744-7429.2004.tb00292.x
Niinemets. (2001). Global-Scale Climatic Controls of Leaf Dry Mass per Area , Density , and Thickness in Trees and Shrubs. 82(2), 453–469.
Niinemets, Ü., Kull, O., & Tenhunen, J. D. (1998). An analysis of light effects on foliar morphology, physiology,. http://treephys.oxfordjournals.org/
Obando-Vargas., F.-V. (2003). No Title. In Árboles del trópico húmedo. Importancia socioeconómica.
Paine, C. E. T., Amissah, L., Auge, H., Baraloto, C., Baruffol, M., Bourland, N., Bruelheide, H., Daïnou, K., de Gouvenain, R. C., Doucet, J. L., Doust, S., Fine, P. V. A., Fortunel, C., Haase, J., Holl, K. D., Jactel, H., Li, X., Kitajima, K., Koricheva, J., … Hector, A. (2015). Globally, functional traits are weak predictors of juvenile tree growth, and we do not know why. Journal of Ecology, 103(4), 978–989. https://doi.org/10.1111/1365-2745.12401
Peel, M. C., Finlayson, B. L., & McMahon, T. A. (2007). Updated world map of the Köppen-Geiger climate classification. Hydrology and Earth System Sciences, 11(5), 1633–1644. https://doi.org/10.5194/hess-11-1633-2007
Pérez-Harguindeguy, N., Díaz, S., Garnier, E., Lavorel, S., Poorter, H., Jaureguiberry, P., Bret-Harte, M. S., Cornwell, W. K., Craine, J. M., Gurvich, D. E., Urcelay, C., Veneklaas, E. J., Reich, P. B., Poorter, L., Wright, I. J., Ray, P., Enrico, L., Pausas, J. G., De Vos, A. C., … Cornelissen, J. H. C. (2013). New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany, 61(3), 167–234. https://doi.org/10.1071/BT12225
Poorter, H., & Evans, J. R. (1998). Photosynthesis Nitrogen Use Efficiecy.Pdf. Springer-Verlag, 26–37.
Poorter, L., Wright, S. J., Paz, H., Ackerly, D. D., Condit, R., Ibarra-Manríquez, G., Harms, K. E., Licona, J. C., Martínez-Ramos, M., Mazer, S. J., Muller-Landau, H. C., Peña-Claros, M., Webb, C. O., & Wright, I. J. (2008). Are functional traits good predictors of demographic rates? Evidence from five neotropical forests. Ecology, 89(7), 1908–1920. https://doi.org/10.1890/07-0207.1
Poorter, Lourens, & Bongers, F. (2006). Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology, 87(7), 1733–1743. https://doi.org/10.1890/0012-9658(2006)87[1733:LTAGPO]2.0.CO;2
Poorter, Lourens, Bongers, L., & Bongers, F. (2006). Architecture of 54 moist-forest tree species: Traits, trade-offs, and functional groups. Ecology, 87(5), 1289–1301. https://doi.org/10.1890/0012-9658(2006)87[1289:AOMTST]2.0.CO;2
Pyšek, P., Richardson, D. M., Pergl, J., Jarošík, V., Sixtová, Z., & Weber, E. (2008). Geographical and taxonomic biases in invasion ecology. Trends in Ecology and Evolution, 23(5), 237–244. https://doi.org/10.1016/j.tree.2008.02.002
Quétier, F., Lavorel, S., Thuiller, W., & Davies, I. (2007). Plant-trait-based modeling assessment of ecosystem-service sensitivity to land-use change. Ecological Applications, 17(8), 2377–2386. https://doi.org/10.1890/06-0750.1
Ramos, R., S. V. & J. U.-G. (2010). Memorias del Taller Nacional Iniciativas para Reducir la Deforestación en la región Andino-Amazónica.
Reich, P. B., Walters, M. B., Tjoelker, M. G., Vanderklein, D., & Buschena, C. (1998). Photosynthesis and respiration rates depend on leaf and root morphology and nitrogen concentration in nine boreal tree species differing in relative growth rate. Functional Ecology, 12(3), 395–405. https://doi.org/10.1046/j.1365-2435.1998.00209.x
Rüger, N., Wirth, C., Wright, S. J., & Condit, R. (2012). Functional traits explain light and size response of growth rates in tropical tree species. Ecology, 93(12), 2626–2636. https://doi.org/10.1890/12-0622.1
Santiago, L. S., Goldstein, G., Meinzer, F. C., Fisher, J. B., Machado, K., Woodruff, D., & Jones, T. (2004). Leaf photosynthetic traits scale with hydraulic conductivity and wood density in Panamanian forest canopy trees. Oecologia, 140(4), 543–550. https://doi.org/10.1007/s00442-004-1624-1
South, D. B. (1995). Relative growth rates: A critique. South African Forestry Journal, 173(1), 43–48. https://doi.org/10.1080/00382167.1995.9629690
Sterck, A. F. J., Poorter, L., Schieving, F., & Sterck, F. J. (2012). The University of Chicago Leaf Traits Determine the Growth ‐ Survival Trade ‐ Off across Rain Forest Tree Species . Reviewed work ( s ): Leaf Traits Determine the Growth-Survival Trade-Off across Rain Forest Tree Species. 167(5), 758–765.
Swenson, N. G., & Enquist, B. J. (2007). Ecological and evolutionary determinants of a key plant functional trait: Wood density and its community-wide variation across latitude and elevation. American Journal of Botany, 94(3), 451–459. https://doi.org/10.3732/ajb.94.3.451
te Beest, M., Esler, K. J., & Richardson, D. M. (2015). Linking functional traits to impacts of invasive plant species: a case study. Plant Ecology, 216(2), 293–305. https://doi.org/10.1007/s11258-014-0437-5
Tecco, P. A., Díaz, S., Cabido, M., & Urcelay, C. (2010). Functional traits of alien plants across contrasting climatic and land-use regimes: Do aliens join the locals or try harder than them? Journal of Ecology, 98(1), 17–27. https://doi.org/10.1111/j.1365-2745.2009.01592.x
Tecco, P. A., Urcelay, C., Díaz, S., Cabido, M., & Pérez-Harguindeguy, N. (2013). Contrasting functional trait syndromes underlay woody alien success in the same ecosystem. Austral Ecology, 38(4), 443–451. https://doi.org/10.1111/j.1442-9993.2012.02428.x
Tomlinson, K. W., Poorter, L., Bongers, F., Borghetti, F., Jacobs, L., & Van Langevelde, F. (2014). Relative growth rate variation of evergreen and deciduous savanna tree species is driven by different traits. Annals of Botany, 114(2), 315–324. https://doi.org/10.1093/aob/mcu107
Uriarte, M., Lasky, J. R., Boukili, V. K., & Chazdon, R. L. (2016). A trait-mediated, neighbourhood approach to quantify climate impacts on successional dynamics of tropical rainforests. Functional Ecology, 30(2), 157–167. https://doi.org/10.1111/1365-2435.12576
Vendramini, F., Díaz, S., Gurvich, D. E., Wilson, P. J., Thompson, K., & Hodgson, J. G. (2002). Leaf traits as indicators of resource-use strategy in floras with succulent species. New Phytologist, 154(1), 147–157. https://doi.org/10.1046/j.1469-8137.2002.00357.x
Villacís, J., Armas, C., Hang, S., & Casanoves, F. (2016a). Selection of Adequate Species for Degraded Areas by Oil-Exploitation Industry as a Key Factor for Recovery Forest in the Ecuadorian Amazon. Land Degradation and Development, 27(7), 1771–1780. https://doi.org/10.1002/ldr.2511
Villacís, J., Casanoves, F., Hang, S., Keesstra, S., & Armas, C. (2016b). Selection of forest species for the rehabilitation of disturbed soils in oil fields in the Ecuadorian Amazon. Science of the Total Environment, 566–567, 761–770. https://doi.org/10.1016/j.scitotenv.2016.05.102
Wiemann, M. C., & Williamson, G. B. (2002). Geographic variation in wood specific gravity: Effects of latitude, temperature, and precipitation. Wood and Fiber Science, 34(1), 96–107.
Wright, I. J., & Cannon, K. (2001). Relationships between leaf lifespan and structural defences in a low-nutrient, sclerophyll flora. Functional Ecology, 15(3), 351–359. https://doi.org/10.1046/j.1365-2435.2001.00522.x
Wright, Ian J., Groom, P. K., Lamont, B. B., Poot, P., Prior, L. D., Reich, P. B., Schulze, E. D., Veneklaas, E. J., & Westoby, M. (2004). Leaf trait relationships in Australian plant species. Functional Plant Biology, 31(5), 551–558. https://doi.org/10.1071/FP03212
##plugins.facebook.comentarios##

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2023 Revista de Biología Tropical