Abstract
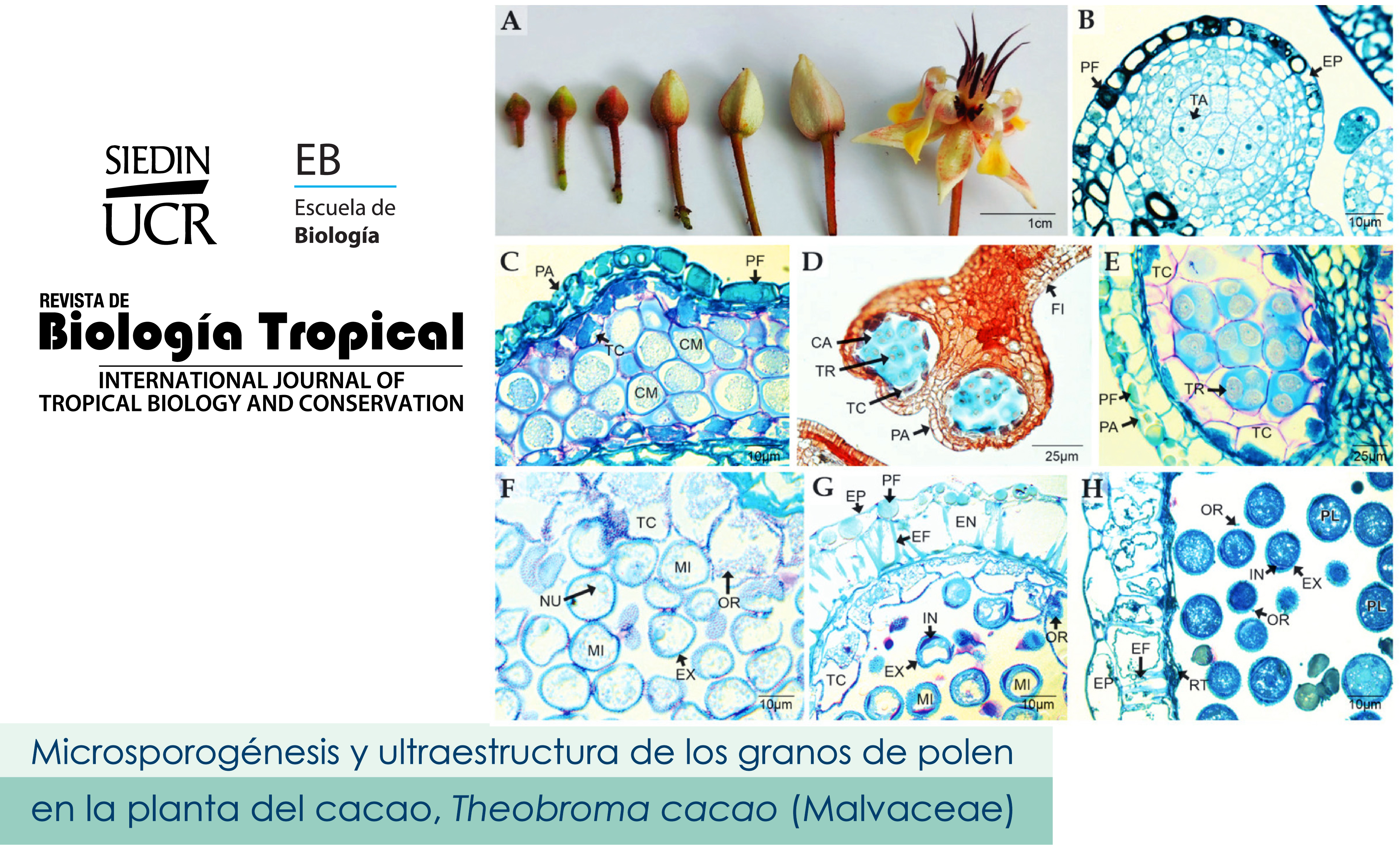
Microsporogenesis and ultrastructure of pollen grains in Thebroma cacao (Malvacea). Introduction: Studies on the microsporogenesis of the chocolate plant are non-existent and little is known about the ultrastructure of the pollen grains. Objectives: the microsporogenesis process is described for the first time and in detail, highlighting ultrastructural aspects of the pollen grains in T. cocao. Methods: More than 30 flowers were processed for each floral development stages according to the protocols for embedding and sectioning in paraffin. The obtained sections were stained with Safranin-Alcian Blue, PAS-Amidoblack and Lacmoid. Additional samples were processed on resin and stained with toluidine blue and ultrathin sections were examined by transmission electron microscopy (TEM). For scanning electron microscopy (SEM) observation, the material was fixed and dehydrated in 2,2-dimethoxypropane, then critically dried and plated with gold. Results: Anthers differentiated by a cellular mass at the ends distal to the staminal filaments. During development, the anthers wall presents several cellular layers and at maturates time; they are reduced to the epidermis and the endothecium. Microspore mother cells divide by mitosis and then undergo meiosis to form tetrads. The tapetum is secretory and remains intact until pollen grains is released, later it degenerates. During sporodermis formation, the exine is first deposited and then the intine. The pollen grains are isopolar, spheroidal, small, tricolpate. The sporodermis is semi-tected, with reticulate ornamentation, heterobrochated reticulum with the muri without ornamentation. The orbicles are individual, smooth and of different sizes. The ultrastructure shows that the pollen grains are semi-tected, the ectexin formed by the tectum, columellae and the basal layer that constitutes the reticulate ornamentation and a very thin and compact endexin. Abundant pollenkitt on the tectum and between the columellae is observed. The intina is very thin, but it develops widely in the colpos areas, forming a compact internal intina and an unusual external intina with a columellated appearance. Conclusion: Anthers structure and development follows the known patterns of angiosperms. Simultaneous microsporogenesis and centripetal deposition of the sporodermis have been previously described for Malvaceae. Intine characters are novel for the Family.
References
Abbott, P. C., Benjamin, T. J., Burniske, G. R., Croft, M. M., Fenton, M., Kelly, C. R., Lundy, M. M., Rodríguez, C. F., & Wilcox, M. D. (2018). An analysis of the supply chain of cacao in Colombia. Purdue University, The Internatiional Center for Tropical Agriculture (CIAT).
Aime, M.C., & Phillips-Mora, W. (2005). The causal agents of witches’ broom and frosty pod rot of cacao (chocolate, Theobroma cacao) form a new lineage of Marasmiaceae. Mycologia, 97(5), 1012–1022.
Alean, J., Chejne, F., Ramírez, S., Rincón, E., Alzate-Arbelaez, A.F., & Rojano, B. (2020). Proposal of a method to evaluate the in-situ oxidation of polyphenolic during the cocoa drying. Drying Technology, 40(3), 559-570.
Ali, Z. A. A. (2020). Taxonomic study of Glossostemon bruguieri desf.(Malvaceae) in Iraq. Plant Archives, 20(2), 926–929.
APG IV. (2016). Chase, M. W., Christenhusz, M. J., Fay, M. F., Byng, J. W., Judd, W. S., ... & Stevens, P. F. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical journal of the Linnean Society, 181(1), 1–20.
Amaral S. J. C. E., Lima, L. L. C. & Saba, M. D. (2017). Pollen morphology of Waltheria L. (Malvaceae-Byttnerioideae) from Bahia, Brazil. Acta Botanica Brasilica, 31(4), 597–612.
Antolinez, S. E. Y. A., Almanza, M. P. J., A., Barona, R. A. F., B., Polanco, D. E. P., & Serrano, C. P. A. (2020). Estado actual de la cacaocultura: una revisión de sus principales limitantes. Revista Ciencia y Agricultura, 17(2), 1–11.
Aoun, M. (2017). Host defense mechanisms during fungal pathogenesis and how these are overcome in suscepti¬ble plants: A review. International Journal of Botany, 13, 82–102.
Bayer, C., Fay, M.F., De bruijn, A.Y., Savolainen, V., Morton, C.M., Kubitzki, K., ... Chase, M.W. (1999). Support for an expanded family concept of Malvaceae within a recircumscribed order Malvales: A combined analysis of plastid atp B and rbc L DNA sequences. Botanical Journal of the Linnean Society, 129(4), 267–303.
Bayer, C., & Kubitzki, K. (2003). Malvaceae. En K. Kubitzki (Ed.), The families and genera of vascular plants (pp. 225–31). Germany: Springer-Verlag.
Beg, M.S., Ahmad, S., Jan, K., & Bashir, K. (2017). Sta¬tus, supply chain and processing of Cocoa-a review. Trends in Food Science & Technology, 66, 108–116.
Bibi, N., Naveed, A., Manzoor, H., & Ajab, K.M. (2010). Systematic implications of pollen morphology in the family Malvaceae from North West frontier province, Pakistan. Pakistan Journal of Botany, 42, 2205-2214.
Blackmore, S., Wortley, A. H., Skvarla, J. J., & Rowley, J. R. (2007). Pollen wall development in flowering plants. New Phytologist, 174(3), 483–498.
Bhattacharjee, R. (2018). Taxonomy and classification of cacao. En P. Umaharan (Ed.), Achieving sustainable cultivation of cocoa (pp. 1–16). Dodds Science Publishing.
Brewbaker, J. (1967). The distribution ann Phylogenetic significance of binucleate and trinucleate pollen grains in the angiosperms. American Journal of Botany, 54 (9): 1069–1083.
Bridgemohan, P., Singh, K., Cazoe, E., Perry, G., Mohamed, A., & Bridgemohan, S.H.R. (2017). Cocoa floral phenology and pollination: Implications for productivity in Caribbean Islands. Journal of Plant Breeding and Crop Science, 9(7), 106–117.
Christensen, P. B. (1986). Pollen morphological studies in Malvaceae. Grana, 25: 95-117.
Crang, R., Lyons-Sobaski, S., & Wise, R. (2018). Plant Anatomy: A Concept-Based Approach to the Structure of Seed Plants. Springer.
Demarco, D. (2017). Histochemical analysis of plant secretory structures. In C. Pellicciari & M. Biggiogera, (Eds.), Histochemistry of single molecules methods and protocols (pp. 313–330). Humana Press.
de Jesus Branco, S.M., da Silva, D.V., Lopes, U.V., & Corrêa, R.X. (2018). Characterization of the sexual self-and cross-compatibility in genotypes of cacao. American Journal of Plant Sciences, 9(9), 1794–1806.
Fernández, G. J., Talle, B., & Wilson, Z. A. (2015). Anther and pollen development: a conserved developmental pathway. Journal of Integrative Plant Biology, 57(11), 876–891.
Furness, C. A., Rudall, P. J., & Sampson, F. B. (2002). Evolution of microsporogenesis in angiosperms. International Journal of Plant Sciences, 163(2), 235–260.
Furness, C. A., & Rudall, P. J. (2004). Pollen aperture evolution–a crucial factor for eudicot success? Trends in Plant Science, 9(3), 154–158.
Galati, B. G., & Rosenfeldt, S. (1998). The pollen development in Ceiba insignis (Kunth) Gibbs and Semir ex Chorisia speciosa St Hil. (Bombacaceae). Phytomorphology, 48(2), 121–129.
Galati, B.G., Monacci, F., Gotelli, M.M., & Rosenfeldt, S. (2007). Pollen, tapetum and orbicule development in Modiolastrum malvifolium (Malvaceae). Annals of Botany, 99(4), 755-763.
Galati, B.G., Gotelli, M.M., Rosenfeldt, S., Torretta, J.P., & Zarlavsky, G. (2011). Orbicules in relation to the pollination modes. En B.J. Kaiser (Ed.), Pollen: structure, y types and effects (pp. 1-15). New York, USA: Nova Science Publisher.
Garcia, T. B., Potiguara, R. C. D. V., Kikuchi, T. Y. S., Demarco, D., & Aguiar-Dias, A. C. A. D. (2014). Leaf anatomical features of three Theobroma species (Malvaceae sl) native to the Brazilian Amazon. Acta Amazonica, 44(3), 291-300.
Halbritter, H. (2017). Guazuma ulmifolia. In: PalDat - A palynological database. https://www.paldat.org/pub/Guazuma_ulmifolia/302981
Halbritter, H., Ulrich, S., Grimsson, F., Weber, M., Zetter, R., Hesse, M., Buchner, R., Svojtka, M., & Frosch-Radivo, A. (2018). Illustrated pollen terminology (2nd Ed.). Springer.
Hamdy, R., & Shamso, E. (2010). Pollen morphology of Sterculiaceae (s. str.) in Egypt and its taxonomic significance. Egyptian Journal of Botany, 50, 103–117.
Heslop Harrison, J. (1966). Cytoplasmatic connections during spore formation in flowering plants. Endeavour 25: 65-72.
Heslop-Harrison J. (1974). The physiology of the pollen grain surface. Proceedings of the Royal Society of London, Series B 190, 275–299.
Heslop-Harrison, Y., & Heslop-Harrison, J. (1982). The microfibrillar component of the pollen intine some structural features. Annals of Botany, 50(6), 831–842.
Lanaud, C., Fouet, O., Legavre, T., Lopes, U., Sounigo, O., Eyango, M.C., ... Gyapay, G. (2017). Deciphering the Theobroma cacao self-incompatibility system: from genomics to diagnostic markers for self-com¬patibility. Journal of Experimental Botany, 68(17), 4775–4790.
Lattar, E. C., Galati, B. G., & Ferrucci, M. S. (2012). Ultrastructural study of pollen and anther development in Luehea divaricata (Malvaceae, Grewioideae) and its systematic implications: Role of tapetal transfer cells, orbicules and male germ unit. Flora-Morphology, Distribution, Functional Ecology of Plants, 207(12), 888–894.
Lattar, E. C., Galati, B. G., & Ferrucci, M. S. (2014). Comparative study of anther development, microsporogenesis and microgametogenesis in species of Corchorus, Heliocarpus, Luehea and Triumfetta (Malvaceae: Grewioideae) from South America. New Zealand Journal of Botany, 52(4), 429–445.
Lattanzio, V., Lattanzio, M.T.V., & Cardinali, A. (2006). Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. En F. Imperato (Ed.), Phytochemistry: Advances in Research (pp. 23-67). India: Research Signpost.
López, H.J.G., López, H.L.E., Avendaño, A.C.H., Aguirre, M.J.F., Espinosa, Z.S., Moreno, M.J.L., ... Suárez, V.G.M. (2018). Biología floral de cacao (Theobroma cacao L.); criollo, trinitario y forastero en México. Agroproductividad, 11(9), 129–136.
Nadot, S., Furness, C. A., Sannier, J., Penet, L., Triki‐Teurtroy, S., Albert, B., & Ressayre, A. (2008). Phylogenetic comparative analysis of microsporogenesis in angiosperms with a focus on monocots. American Journal of Botany, 95(11), 1426–1436.
Narayanapur, V.B., Suma, B., & Minimol, J.S. (2018). Self-incompatibility: a pollination control mechanism in plants. International Journal of Plant Sciences (Muzaffarnagar), 13(1), 201–212.
N’Zi, J.C., Kahia, J., Diby, L., & Kouamé, C. (2017). Compatibility of ten elite cocoa (Theobroma cacao L.) clones. Horticulturae, 3(3), 1–8.
Osorio, G.J.A., Berdugo, C.J., Coronado, R.A., Zapata, Y.P., Quintero, C., Gallego, S.G., & Yockteng, R. (2017). Colombia a source of cacao genetic diversity as revealed by the population structure analysis of germplasm bank of Theobroma cacao L. Frontiers in Plant Science, 8, 1–13.
Pacini, E. (2010). Relationships between tapetum, loculus, and pollen during development. International Journal of Plant Sciences, 171(1), 1–11.
Pacini, E., & Hesse, M. (2012). Uncommon pollen walls: reasons and consequences. Verhandlungen der Zoologisch-Botanischen Gesellschaft in Osterreich, 148, 291–306.
Perveen, A., & Qaiser, M. (2009). Pollen flora of Pakistan- Malvaceae: Dombeyoideae-Lxii. Pakistan Journal of Botany, 41(2), 491–494.
Prieu, C., Toghranegar, Z., Gouyon, P. H., & Albert, B. (2019). Microsporogenesis in angiosperms producing pantoporate pollen. Botany Letters, 166(4), 457–466.
Punt, W., Hoen, P. P., Blackmore, S., Nilsson, S., & Le Thomas, A. (2007). Glossary of pollen and spore terminology. Review of Palaeobotany and Palynology, 143(1-2), 1–83.
Ramming, D.W., Hinrichs, H.A., & Richardson, P.E. (1973). Sequential staining of callose by aniline blue and lacmoid for fluorescence and regular microscopy on a durable preparation of the same specimen. Stain Technology, 48(3), 133–134.
Rangel, F.M.A., Zavaleta, M.H.A., Córdova, T.L., López, A.A.P., Delgado, A.A., Vidales, F.I., & Villegas, M.Á. (2012). Anatomía e histoquímica de la semilla del cacao (Theobroma cacao L.) criollo mexicano. Revista Fitotecnia Mexicana, 35(3), 189–197.
Richardson, J.E., Whitlock, B.A., Meerow, A.W., & Madri¬ñán, S. (2015). The age of chocolate: a diversification history of Theobroma and Malvaceae. Frontiers in Ecology and Evolution, 3, 1–14.
Rincón-Barón, E. J., Grisales, E. C., Cuaran, V. L., & Cardona, N. L. (2020). Alteraciones anatómicas e histoquímicas ocasionadas por la oidiosis en hojas de Hydrangea macrophylla (Hydrangeaceae). Revista de Biología Tropical, 68(3), 959–976.
Rincón-Baron, E. J., Zarate, D. A., Castañeda, G. A. A., Cuarán, V. L., & Passarelli, L. M. (2021a). Micromorfología y ultraestructura de las anteras y los granos de polen en diez genotipos élite de Theobroma cacao (Malvaceae). Revista de Biología Tropical, 69(2), 403–421.
Rincón-Barón, E. J., Torres-Rodríguez, G. A., Passarelli, L. M., Zárate, D. A., Cuarán, V. L., & Plata-Arboleda, S. (2021 b). Microsporogénesis y micromorfología del polen de la planta Alcea rosea (Malvaceae). Revista de Biología Tropical, 69(3), 852–864.
Ruggiero, F., & Bedini, G. (2020). Phylogenetic and morphologic survey of orbicules in angiosperms. Taxon, 69(3), 543-566.
Ruzin, S. E. (1999). Plant microtechnique and microscopy. Oxford University.
Saba, M. D., & dos Santos, F. D. A. R. (2015). Pollen morphology and exine ultrastructure of selected species of Waltheria L. (Byttnerioideae-Malvaceae). Review of Palaeobotany and Palynology, 221, 204-210.
Sánchez, V., Zambrano J. L., Iglesias, C., Rodríguez, E., Villalobos, V., Díaz F. J., Carrillo, N., Gutiérrez, A., Camacho, A., & Rodríguez, O. (2019). La cadena de valor del cacao en América Latina y el Caribe. FONTAGRO, ESPOL, INIAP. https://www.researchgate.net/publication/341051482_La_cadena_de_valor_del_cacao_en_America_Latina_y_el_Caribe.
Scott, R. J., Spielman, M., & Dickinson, H. G. (2004). Stamen structure and function. The Plant Cell, 16(S1), S46–S60
Squicciarini, M.P., & Swinnen, J. (2016). The Economics of Chocolate. Oxford, UK: Oxford University Press.
Sivachandran, R., Gnanam, R., Sudhakar, D., Suresh, J., & Ram, S.G. (2017). Influence of genotypes, stages of microspore, pre-treatments and media fac¬tors on induction of callus from anthers of cocoa (Theobroma cacao L.). Journal of Plantation Crops, 45(3),162–172.
Soukup, A. (2014). Selected simple methods of plant cell wall histochemistry and staining for light microscopy. En V. Žárský, & F. Cvrčková (Eds.), Plant cell morphogenesis: methods and protocols, methods in molecular biology (pp. 25–40). New York, USA: Humana Press.
Strittmatter, L. I, Galati, B. G., & Monacci, F. (2000). Ubisch bodies in the peritapetal membrane of Abutilon pictum Gill (Malvaceae). Beiträge zur Biologie der Pflanzen, 71, 1-10.
Strittmatter, L.I., & Galati, B.G. (2001). Development of anthers, microsporogenesis and microgametogenesis of Myosotis azorica and M. laxa (Boraginaceae). Phytomorphology, 51(1), 1-10.
Swanson, J.D., Carlson, J.E., & Guiltinan, M.J. (2008). Comparative flower development in Theobroma cacao based on temporal morphological indicators. International Journal of Plant Sciences, 169(9), 1187–1199.
Tang, Y. A., Gao, H. U. I., Wang, C. M., & Chen, J. Z. (2006). Microsporogenesis and microgametogenesis of Excentrodendron hsienmu (Malvaceae sl) and their systematic implications. Botanical Journal of the Linnean Society, 150(4), 447–457.
Tang, Y. A., Gao, H., & Xie, J. Z. (2009). An embryological study of Eriolaena candollei Wallich (Malvaceae) and its systematic implications. Flora, 204(8), 569–580.
Varela, M. C., Arslan, I., Reginato, M. A., Cenzano, A. M., Luna, M. V. (2016). Phenolic compounds as indicators of drought resistance in shrubs from Patagonian shrublands (Argentina). Plant Physiology and Biochemistry 104, 81–91.
Venancio, L. A. C. de Souza, N. C., Saba, D. M., & Custódio G., E. 2022. Pollen morphology of Malvaceae s.l. from Cerrado Forest Fragments: details of aperture and ornamentation in the pollen types definition. Palynology, 46 (1), 1–15.
Verstraete, B., Moon, H.K., Smets, E., & Huysmans, S. (2014). Orbicules in flowering plants: a phylogenetic perspective on their form and function. The Botanical Review, 80(2), 107-134.
Von Balthazar, M., Schönenberger, J., Alverson, W. S., Janka, H., Bayer, C., & Baum, D. A. (2006). Structure and evolution of the androecium in the Malvatheca clade (Malvaceae sl) and implications for Malvaceae and Malvales. Plant Systematics and Evolution, 260(2), 171–197.
Wickramasuriya, A.M., & Dunwell, J.M. (2018). Cacao biotechnology: current status and future prospects. Plant Biotechnology Journal, 16(1), 4–17.
Young, A. M., Erickson, E. H., Strand, M. A., & Erickson, B. J. (1987). Pollination biology of Theobroma and Herrania (Sterculiaceae)—I. Floral biology. International Journal of Tropical Insect Science, 8(2), 151–164.
##plugins.facebook.comentarios##

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2023 Revista de Biología Tropical