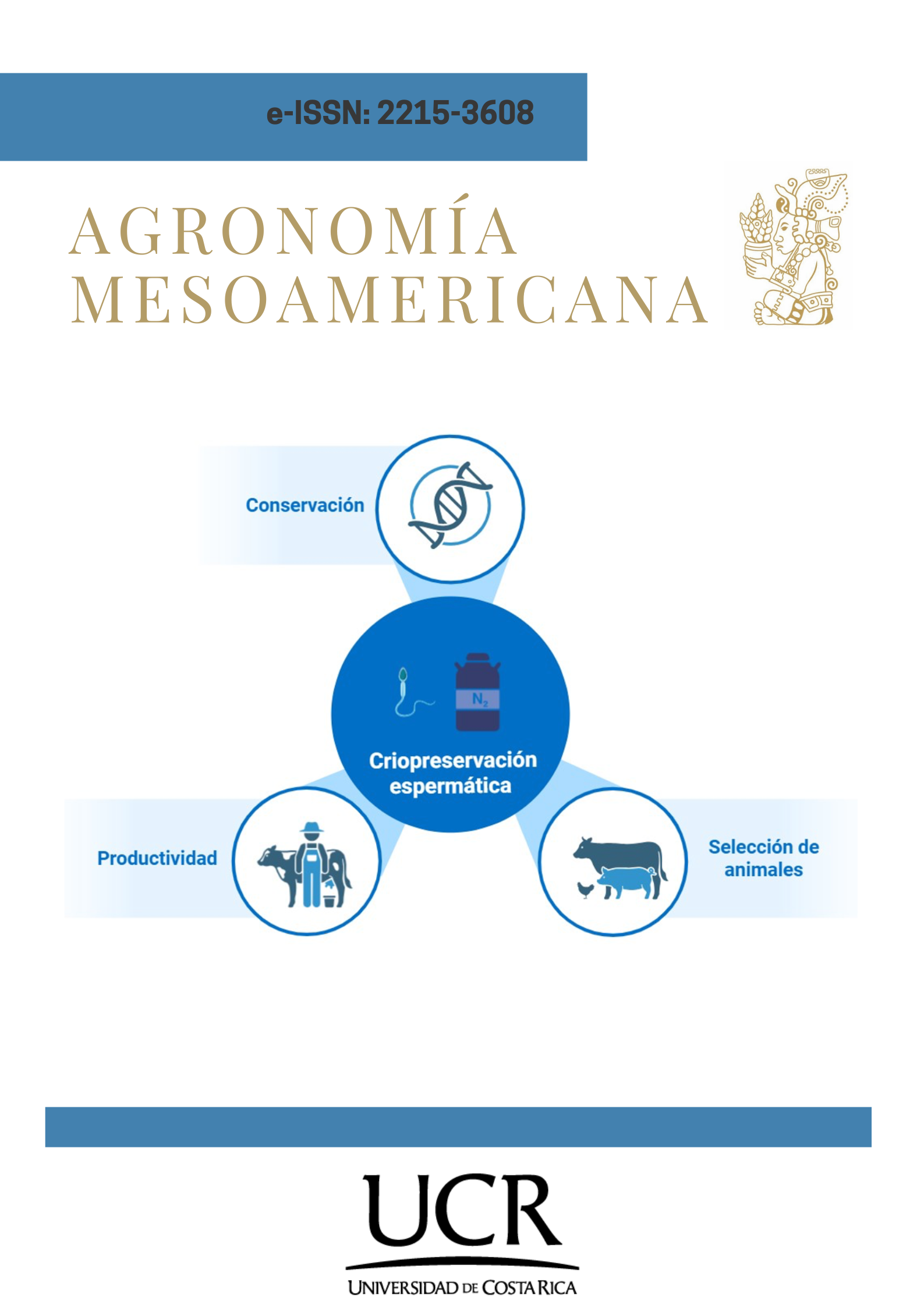
La criopreservación del germoplasma de especies ganaderas: Un paso hacia la sostenibilidad
DOI:
https://doi.org/10.15517/am.2025.61375Palabras clave:
antioxidantes, centrifugación, diluyentes, conservación de biodiversidad, especies reactivas de oxígenoResumen
Introducción. El cambio climático ha conllevado a la necesidad de modificar la forma de producción en los sistemas ganaderos. Objetivo. Revisar el estado del arte sobre la criopreservación de espermatozoides de especies ganaderas y su posible contribución al desarrollo sostenible. Desarrollo. Se revisaron artículos científicos publicados entre los años 2000 y 2024 en las bases de datos Web of Science, Scopus y Science Direct. La congelación espermática se puede considerar una forma de optimizar la reproducción de los animales. Sin embargo, durante el proceso, se puede estimular la formación de especies reactivas de oxígeno, que promueven la peroxidación lipídica de la membrana, lo que puede generar daños a nivel estructural y molecular que comprometen la funcionalidad espermática y la capacidad fecundante del gameto masculino. El éxito de la criopreservación de los espermatozoides de especies ganaderas puede ser mejorado mediante la inclusión de factores extrínsecos como la adición de antioxidantes, la centrifugación o la selección del tipo de congelación. Esta biotecnología reproductiva se asocia con la inseminación artificial, y la combinación de estas técnicas ha permitido optimizar la rentabilidad de los sistemas ganaderos a través de la mejora genética continua. Conclusiones. La optimización de la criopreservación del germoplasma de las especies de interés zootécnico ha contribuido al aumento de la productividad y la eficiencia de los sistemas ganaderos, así como a la posibilidad de conservación de las especies, los cuales son factores claves para alcanzar la sostenibilidad.
Descargas
Citas
Agarwal, A., Varghese, A. C., & Sharma, R. K. (2009). Markers of oxidative stress and sperm chromatin integrity. In O.-K. Park-Sarge, & T. E. Curry (Eds.), Methods in molecular biology (pp. 377–402). https://doi.org/10.1007/978-1-60327-378-7_24
Aitken, R. J., & Baker, M. A. (2006). Oxidative stress, sperm survival and fertility control. Molecular and Cellular Endocrinology, 250(1–2), 66–69. https://doi.org/10.1016/j.mce.2005.12.026
Akhter, S., Ansari, M. S., Rakha, B. A., Ullah, N., Andrabi, S. M. H., & Khalid, M. (2011). In Vitro Evaluation of liquid-stored buffalo semen at 5°C diluted in soya lecithin based extender (Bioxcell®), Tris-citric egg yolk, skim milk and egg yolk-citrate extenders. Reproduction in Domestic Animals, 46(1), 45–49. https://doi.org/10.1111/j.1439-0531.2009.01561.x
Akhter, S., Zubair, M., Mahmood, M., Andrabi, S. M. H., Hameed, N., Ahmad, E., & Saleemi, M. K. (2023). Effects of vitamins C and E in tris citric acid glucose extender on chilled semen quality of Kail ram during different storage times. Scientific Reports, 13(1), Article 18123. https://doi.org/10.1038/s41598-023-43831-2
Almubarak, A. M., Kim, W., Abdelbagi, N. H., Balla, S. E., Yu, I.-J., & Jeon, Y. (2021). Washing solution and centrifugation affect kinematics of cryopreserved boar semen. Journal of Animal Reproduction and Biotechnology, 36(2), 65–75. https://doi.org/10.12750/jarb.36.2.69
Androni, D. A., Dodds, S., Tomlinson, M., & Maalouf, W. E. (2021). Is pre-freeze sperm preparation more advantageous than post-freeze? Reproduction and Fertility, 2(1), 17–25. https://doi.org/10.1530/RAF-20-0041
Araya-Zúñiga, I., Sevilla, F., Barquero, V., & Valverde, A. (2023). The effect of extender, age, and bovine sexual status on the sperm kinematics. Agronomía Mesoamericana, 34(3), Article 52597. https://doi.org/10.15517/am.2023.52597
Arnold, D. M., Gray, C., Roth, T. L., Mitchell, S., & Graham, L. H. (2017). A simple, field-friendly technique for cryopreserving semen from Asian elephants (Elephas maximus). Animal Reproduction Science, 182, 84–94. https://doi.org/10.1016/j.anireprosci.2017.05.003
Atig, F., Kerkeni, A., Saad, A., & Ajina, M. (2017). Effects of reduced seminal enzymatic antioxidants on sperm DNA fragmentation and semen quality of Tunisian infertile men. Journal of Assisted Reproduction and Genetics, 34(3), 373–381. https://doi.org/10.1007/s10815-013-9936-x
Branco, C. S., Garcez, M. E., Pasqualotto, F. F., Erdtman, B., & Salvador, M. (2010). Resveratrol and ascorbic acid prevent DNA damage induced by cryopreservation in human semen. Cryobiology, 60(2), 235–237. https://doi.org/10.1016/j.cryobiol.2009.10.012
Brugnon, F., Ouchchane, L., Pons-Rejraji, H., Artonne, C., Farigoule, M., & Janny, L. (2013). Density gradient centrifugation prior to cryopreservation and hypotaurine supplementation improve post-thaw quality of sperm from infertile men with oligoasthenoteratozoospermia. Human Reproduction, 28(8), 2045–2057. https://doi.org/10.1093/humrep/det253
Bustani, G. S., & Baiee, F. H. (2021). Semen extenders: An evaluative overview of preservative mechanisms of semen and semen extenders. Veterinary World, 14(5), 1220–1233. https://doi.org/10.14202/vetworld.2021.1220-1233
Büyükleblebici, S., Tuncer, P. B., Bucak, M. N., Eken, A., Sariözkan, S., Taşdemir, U., & Endirlik, B. Ü. (2014). Cryopreservation of bull sperm: Effects of extender supplemented with different cryoprotectants and antioxidants on sperm motility, antioxidant capacity and fertility results. Animal Reproduction Science, 150(3–4), 77–83. https://doi.org/10.1016/j.anireprosci.2014.09.006
Carriço, C., Barbas, J. P., Pimenta, J., & Simões, J. (2023). Effect of in vitro addition of melatonin and glutathione on seminal parameters of rams in diluted semen and after thawing. Veterinary Sciences, 10(7), Article 446. https://doi.org/10.3390/vetsci10070446
Castillo, A., Lenzi, C., Pirone, A., Baglini, A., Cerolini, S., Iaffaldano, N., Sartore, S., Russo, C., Schiavone, A., & Di Cossato, M. M. F. (2021). Optimization of a protocol for the cryopreservation of sperm in pellets for the common pheasant (Phasianus colchicus mongolicus). Animals, 11(8), Article 2472. https://doi.org/10.3390/ani11082472
Catalán, J., Yánez-Ortiz, I., Torres-Garrido, M., Ribas-Maynou, J., Llavanera, M., Barranco, I., Yeste, M., & Miró, J. (2024). Impact of seminal plasma antioxidants on DNA fragmentation and lipid peroxidation of frozen–thawed horse sperm. Antioxidants, 13(3), Article 322. https://doi.org/10.3390/antiox13030322
Catalán, J., Yánez-Ortiz, I., Tvarijonaviciute, A., González-Arostegui, L. G., Rubio, C. P., Yeste, M., Miró, J., & Barranco, I. (2022). Impact of seminal plasma antioxidants on donkey sperm cryotolerance. Antioxidants, 11(2), Article 417. https://doi.org/10.3390/antiox11020417
ChaithraShree, A. R., Ingole, S. D., Dighe, V. D., Nagvekar, A. S., Bharucha, S. V., Dagli, N. R., Kekan, P. M., & Kharde, S. D. (2020). Effect of melatonin on bovine sperm characteristics and ultrastructure changes following cryopreservation. Veterinary Medicine and Science, 6(2), 177–186. https://doi.org/10.1002/vms3.224
Champroux, A., Torres-Carreira, J., Gharagozloo, P., Drevet, J. R., & Kocer, A. (2016). Mammalian sperm nuclear organization: Resiliencies and vulnerabilities. Basic and Clinical Andrology, 26, Article 17. https://doi.org/10.1186/s12610-016-0044-5
Chung, E. L. T., Nayan, N., Nasir, N. S. M., Hing, P. S. A., Ramli, S., Rahman, M. H. A., & Kamalludin, M. H. (2019). Effect of honey as an additive for cryopreservation on bull semen quality from different cattle breeds under tropical condition. Journal of Animal Health and Production, 7(4), 171–178. https://doi.org/10.17582/journal.jahp/2019/7.4.171.178
Cojkic, A., Hansson, I., Johannisson, A., Axner, E., & Morrell, J. M. (2024). Single layer centrifugation as a method for bacterial reduction in bull semen for assisted reproduction. Veterinary Research Communications, 48(1), 39–48. https://doi.org/10.1007/s11259-023-10178-y
Córdova-Izquierdo, A., Saltijeral Oaxaca, J., Ruiz Lang, G., Xolalpa Campos, V., Cortés Suárez, S., Peña Betancourt, S. D., Córdova-Jiménez, C., Córdova-Jiménez, M., Méndez Mendoza, M., Crispín Huerta, R., Juárez Mosqueda, M., & Guerra Liera, J. E. (2010). Estrés oxidativo en gametos. Revista Electronica Veterinaria, 11(7), 1–32. https://www.veterinaria.org/index.php/REDVET/issue/view/1
Curry, M. R. (2000). Cryopreservation of semen from domestic livestock. Reviews of Reproduction, 5, 46–52.
Da Silva, A. (2014). El plan de acción mundial de la FAO sobre los recursos zoogenéticos y su aplicación en Latinoamérica y el Caribe. Revista Cubana de Ciencia Agrícola, 48(1), 35–41.
Di Iorio, M., Esposito, S., Rusco, G., Roncarati, A., Miranda, M., Gibertoni, P. P., Cerolini, S., & Iaffaldano, N. (2019). Semen cryopreservation for the Mediterranean brown trout of the Biferno River (Molise-Italy): Comparative study on the effects of basic extenders and cryoprotectants. Scientific Reports, 9, Article 9703. https://doi.org/10.1038/s41598-019-45006-4
Ellerbrock, R. E., Prell, M. J., Stewart, J. L., Bojko, M. S., Lima, F. S., & Canisso, I. F. (2017). Comparison of Centrifugation and Noncentrifugation Methods to Cryopreserve Stallion Epididymal Semen. Journal of Equine Veterinary Science, 50, 27–32. https://doi.org/10.1016/j.jevs.2016.11.005
El-Seadawy, I. E., Kotp, M. S., El-Maaty, A. M. A., Fadl, A. M., El-Sherbiny, H. R., & Abdelnaby, E. A. (2022). The impact of varying doses of moringa leaf methanolic extract supplementation in the cryopreservation media on sperm quality, oxidants, and antioxidant capacity of frozen-thawed ram sperm. Tropical Animal Health and Production, 54(6), Article 344. https://doi.org/10.1007/s11250-022-03344-y
Esteso, M. C., Soler, A. J., Fernández-Santos, M. R., Quintero-Moreno, A. A., & Garde, J. J. (2006). Functional significance of the sperm head morphometric size and shape for determining freezability in Iberian red deer (Cervus elaphus hispanicus) epididymal sperm samples. Journal of Andrology, 27(5), 662–670. https://doi.org/10.2164/jandrol.106.000489
Félix, F., Oliveira, C. C. V., & Cabrita, E. (2021). Antioxidants in fish sperm and the potential role of melatonin. Antioxidants, 10(1), Article 36. https://doi.org/10.3390/antiox10010036
Ford, W. C. L. (2004). Regulation of sperm function by reactive oxygen species. Human Reproduction Update, 10(5), 387–399. https://doi.org/10.1093/humupd/dmh034
Fumuso, F. G., Bertuzzi, M. L., Velásquez González, N., Miragaya, M. H., & Carretero, M. I. (2021). Cryopreservation of llama semen using a combination of permeable cryoprotectants. Reproduction in Domestic Animals, 56(7), 958–964. https://doi.org/10.1111/rda.13937
García, W., Maxi, E., Macedo, V., Ampuero, E., & Malaga, J. (2021). Cryopreservation of alpaca spermatozoa obtained via post copula in a tris extender with egg yolk from three avian species. Spermova, 11(1), 11–16. https://doi.org/10.18548/ASPE/0009.02
Gloria, A., Carluccio, A., Wegher, L., Robbe, D., Befacchia, G., & Contri, A. (2016). Single and double layer centrifugation improve the quality of cryopreserved bovine sperm from poor quality ejaculates. Journal of Animal Science and Biotechnology, 7, Article 30. https://doi.org/10.1186/s40104-016-0088-6
Gomes-Alves, S., Alvarez, M., Nicolas, M., Lopez -Urueña, E., Martínez-Rodríguez, C., Borragan, S., de Paz, P., & Anel, L. (2014). Use of commercial extenders and alternatives to prevent sperm agglutination for cryopreservation of brown bear semen. Theriogenology, 82(3), 469–474. https://doi.org/10.1016/j.theriogenology.2014.05.015
Gül, A., Şahinler, N., Onal, A. G., Hopkins, B. K., & Sheppard, W. S. (2017). Effects of diluents and plasma on honey bee (Apis mellifera L.) drone frozen-thawed semen fertility. Theriogenology, 101, 109–113. https://doi.org/10.1016/j.theriogenology.2017.06.020
Gulov, A. N., Berezin, A. S., Larkina, E. O., Saltykova, E. S., & Kaskinova, M. D. (2023). Creation of a biobank of the sperm of the honey bee drones of different subspecies of Apis mellifera L. Animals, 13(23), Article 3684. https://doi.org/10.3390/ani13233684
Gürler, H., Malama, E., Heppelmann, M., Calisici, O., Leiding, C., Kastelic, J. P., & Bollwein, H. (2016). Effects of cryopreservation on sperm viability, synthesis of reactive oxygen species, and DNA damage of bovine sperm. Theriogenology, 86(2), 562–571. https://doi.org/10.1016/j.theriogenology.2016.02.007
Gutiérrez-Cepeda, L., Fernández, A., Crespo, F., Ramírez, M. Á., Gosálvez, J., & Serres, C. (2012). The effect of two pre-cryopreservation single layer colloidal centrifugation protocols in combination with different freezing extenders on the fragmentation dynamics of thawed equine sperm DNA. Acta Veterinaria Scandinavica, 54, Article 72. https://doi.org/10.1186/1751-0147-54-72
He, W.-H., Zhai, X.-H., Duan, X.-J., & Di, H.-S. (2020). Effect of resveratrol treatment on apoptosis and apoptotic pathways during boar semen freezing. Journal of Zhejiang University: Science B (Biomedicine & Biotechnology), 21(6), 485–494. https://doi.org/10.1631/jzus.B1900520
Hermes, R., Hildebrandt, T. B., & Göritz, F. (2018). Cryopreservation in rhinoceros—setting a new benchmark for sperm cryosurvival. PLoS ONE, 13(7), Article e0200154. https://doi.org/10.1371/journal.pone.0200154
Hezavehei, M., Sharafi, M., Kouchesfahani, H. M., Henkel, R., Agarwal, A., Esmaeili, V., & Shahverdi, A. (2018). Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reproductive BioMedicine Online, 37(3), 327–339. https://doi.org/10.1016/j.rbmo.2018.05.012
Hidalgo, M., Diaz-Jimenez, M., Consuegra, C., Pereira, B., & Dorado, J. (2020). Vitrification of donkey sperm: Is it better using permeable cryoprotectants? Animals, 10(9), Article 1462. https://doi.org/10.3390/ani10091462
Hoogewijs, M., Morrell, J., Van Soom, A., Govaere, J., Johannisson, A., Piepers, S., De Schauwer, C., De Kruif, A., & De Vliegher, S. (2011). Sperm selection using single layer centrifugation prior to cryopreservation can increase thawed sperm quality in stallions. Equine Veterinary Journal, 43(Supp. 40), 35–41. https://doi.org/10.1111/j.2042-3306.2011.00489.x
Hristov, A. N., Ott, T., Tricarico, J., Rotz, A., Waghorn, G., Adesogan, A., Dijkstra, J., Montes, F., Oh, J., Kebreab, E., Oosting, S. J., Gerber, P. J., Henderson, B., Makkar, H. P. S., & Firkins, J. L. (2013). Special Topics — Mitigation of methane and nitrous oxide emissions from animal operations: III. A review of animal management mitigation options. Journal of Animal Science, 91(11), 5095–5113. https://doi.org/10.2527/jas.2013-6585
Hungerford, A., Bakos, H. W., & Aitken, R. J. (2023). Sperm cryopreservation: current status and future developments. Reproduction, Fertility, and Development, 35(3), 265–281. https://doi.org/10.1071/RD22219
Ibrahim, S., Talha, N. A. H., Kim, J., Jeon, Y., & Yu, I. (2022). Cryopreservation of Siberian tiger (Panthera tigris altaica) epididymal spermatozoa: pilot study of post-thaw sperm characteristics. Journal of Animal Reproduction and Biotechnology, 37(2), 130–135. https://doi.org/10.12750/jarb.37.2.130
Kaeoket, K., & Chanapiwat, P. (2023). The beneficial effect of resveratrol on the quality of frozen-thawed boar sperm. Animals, 13(18), Article 2829. https://doi.org/10.3390/ani13182829
Kajabova, S., Silva, H., Valadao, L., & Moreira da Silva, F. (2020). Artificial insemination and cryopreservation of boar semen: current state and problematics. Open Science Journal, 5(2), 1–12. https://osjournal.org/ojs/index.php/OSJ/article/view/2353/0
Kalwar, Q., Chu, M., Korejo, R. A., Soomro, H., & Yan, P. (2022). Cryopreservation of yak semen: A comprehensive review. Animals, 12(24), Article 3451. https://doi.org/10.3390/ani12243451
Khan, I. M., Cao, Z., Liu, H., Khan, A., Rahman, S. U., Khan, M. Z., Sathanawongs, A., & Zhang, Y. (2021). Impact of cryopreservation on spermatozoa freeze-thawed traits and relevance OMICS to assess sperm cryo-tolerance in farm animals. Frontiers in Veterinary Science, 8, Article 609180. https://doi.org/10.3389/fvets.2021.609180
Li, J., Barranco, I., Tvarijonaviciute, A., Molina, M. F., Martinez, E. A., Rodriguez-Martinez, H., Parrilla, I., & Roca, J. (2018). Seminal plasma antioxidants are directly involved in boar sperm cryotolerance. Theriogenology, 107, 27–35. https://doi.org/10.1016/j.theriogenology.2017.10.035
Li, C. Y., Zhao, Y. H., Hao, H. S., Wang, H. Y., Huang, J. M., Yan, C. L., Du, W. H., Pang, Y. W., Zhang, P. P., Liu, Y., Zhu, H. Bin, & Zhao, X. M. (2018). Resveratrol significantly improves the fertilisation capacity of bovine sex-sorted semen by inhibiting apoptosis and lipid peroxidation. Scientific Reports, 8, Article 7603. https://doi.org/10.1038/s41598-018-25687-z
Lima-Verde, I., Hurri, E., Ntallaris, T., Johannisson, A., Stålhammar, H., & Morrell, J. M. (2022). Sperm quality in young bull semen can be improved by single layer centrifugation. Animals, 12(18), Article 2435. https://doi.org/10.3390/ani12182435
Lima-Verde, I. B., Johannisson, A., Ntallaris, T., Al-Essawe, E., Al-Kass, Z., Nongbua, T., Dórea, F., Lundeheim, N., Kupisiewicz, K., Edman, A., & Morrell, J. M. (2018). Effect of freezing bull semen in two non-egg yolk extenders on post-thaw sperm quality. Reproduction in Domestic Animals, 53(1), 127–136. https://doi.org/10.1111/rda.13080
Madhusoodan, A. P., Sejian, V., Rashamol, V. P., Savitha, S. T., Bagath, M., Krishnan, G., & Bhatta, R. (2019). Resilient capacity of cattle to environmental challenges – An updated review. Journal of Animal Behaviour and Biometeorology, 7(3), 104–118. https://doi.org/10.31893/2318-1265jabb.v7n3p104-118
Makris, A., Alevra, A. I., Exadactylos, A., & Papadopoulos, S. (2023). The role of melatonin to ameliorate oxidative stress in sperm cells. International Journal of Molecular Sciences, 24(20), Article 15056. https://doi.org/10.3390/ijms242015056
Malo, C., Crichton, E. G., & Skidmore, J. A. (2020). Preservation of the spermatozoa of the dromedary camel (Camelus dromedarius) by chilling and freezing: The effects of cooling time, extender composition and catalase supplementation. Theriogenology, 153, 9–18. https://doi.org/10.1016/j.theriogenology.2020.04.043
Malvezzi, H., Sharma, R., Agarwal, A., Abuzenadah, A. M., & Abu-Elmagd, M. (2014). Sperm quality after density gradient centrifugation with three commercially available media: A controlled trial. Reproductive Biology and Endocrinology, 12, Article 121. https://doi.org/10.1186/1477-7827-12-121
Maroto-Morales, A., García-Álvarez, O., Ramón, M., Martínez-Pastor, F., Fernández-Santos, M. R., Soler, A. J., & Garde, J. J. (2016). Current status and potential of morphometric sperm analysis. Asian Journal of Andrology, 18(6), 863–870. https://doi.org/10.4103/1008-682X.187581
Marques, J. C. C., Cezar, A. R. R., do Nascimento, A. D., da Silva, J. P., Batista, A. M., Guerra, M. M. P., & Câmara, D. R. (2023). Relationship between Na/K-ATPase in thawed sperm and fertility of Angus bulls. Animal Reproduction, 20(4), Article e20220066. https://doi.org/10.1590/1984-3143-AR2022-0066
Martín, A., Castaño, C., O’Brien, E., Toledano-Díaz, A., Guerra, R., Gómez-Guillamón, F., & Santiago-Moreno, J. (2023). Equilibration time improves the sperm variables of wild ruminant ejaculated and epididymal sperm cryopreserved by ultra-rapid freezing. Cryobiology, 113, Article 104579. https://doi.org/10.1016/j.cryobiol.2023.104579
Martínez-Aguilar, E. A. (2020). Reseña del origen y desaparición de los bovinos criollos en El Salvador, el primer paso para una posible reintroducción. Revista Científica de La Facultad de Ciencias Agronómicas de La Universidad de El Salvador, 3(16), 118–129. https://doi.org/10.5281/zenodo.10840488
Martinez-Alborcia, M. J., Valverde, A., Parrilla, I., Vazquez, J. M., Martinez, E. A., & Roca, J. (2012). Detrimental effects of non-functional spermatozoa on the freezability of functional spermatozoa from boar ejaculate. PLoS ONE, 7(5), Article e36550. https://doi.org/10.1371/journal.pone.0036550
Masoudi, R., Zare Shahneh, A., Towhidi, A., Kohram, H., Akbarisharif, A., & Sharafi, M. (2017). Fertility response of artificial insemination methods in sheep with fresh and frozen-thawed semen. Cryobiology, 74, 77–80. https://doi.org/10.1016/j.cryobiol.2016.11.012
Medina-Robles, V. M., Sánchez-Carvajal, E., Velasco-Santamaria, Y. M., & Cruz-Casallas, P. E. (2007). Crioconservación de semen bovino usando un congelador programable (CL-8800) y determinación de su calidad postdescongelación por medio un sistema de análisis espermático asistido por computador (CASA). Revista ORINOQUIA, 11(1), 75–86.
Mirkena, T., Duguma, G., Haile, A., Tibbo, M., Okeyo, A. M., Wurzinger, M., & Sölkner, J. (2010). Genetics of adaptation in domestic farm animals: a review. Livestock Science, 132(1–3), 1–12. https://doi.org/10.1016/j.livsci.2010.05.003
Moore, S. G., & Hasler, J. F. (2017). A 100-Year Review: Reproductive technologies in dairy science. Journal of Dairy Science, 100(12), 10314–10331. https://doi.org/10.3168/jds.2017-13138
Morrell, J. M., & Rodriguez-Martinez, H. (2016). Colloid centrifugation of semen: applications in assisted reproduction. American Journal of Analytical Chemistry, 7(8), 597–610. https://doi.org/10.4236/ajac.2016.78055
Mphaphathi, M. L., & Nedambale, T. L. (2021). Effect of repeated freezing and thawing on nguni sperm parameters evaluated by computer assisted sperm analyzer system. American Journal of Animal and Veterinary Sciences, 16(1), 1–6. https://doi.org/10.3844/ajavsp.2021.1.6
Mujica, F. (2009). Diversidad y conservación de los recursos zoogenéticos de país. Agro Sur, 37(3), 134–175.
Nagata, M. P. B., Egashira, J., Katafuchi, N., Endo, K., Ogata, K., Yamanaka, K., Yamanouchi, T., Matsuda, H., Hashiyada, Y., & Yamashita, K. (2019). Bovine sperm selection procedure prior to cryopreservation for improvement of post-thawed semen quality and fertility. Journal of Animal Science and Biotechnology, 10, Article 91. https://doi.org/10.1186/s40104-019-0395-9
Ogata, K., Imai, A., Sato, S., Nishino, K., Watanabe, S., Somfai, T., Kobayashi, E., & Takeda, K. (2022). Effects of reduced glutathione supplementation in semen freezing extender on frozen-thawed bull semen and in vitro fertilization. Journal of Reproduction and Development, 68(1), 53–61.
Ozimic, S., Ban-Frangez, H., & Stimpfel, M. (2023). Sperm cryopreservation today: Approaches, efficiency, and pitfalls. Current Issues in Molecular Biology, 45(6), 4716–4734. https://doi.org/10.3390/cimb45060300
Phaniendra, A., Jestadi, D. B., & Periyasamy, L. (2015). Free radicals: Properties, sources, targets, and their implication in various diseases. Indian Journal of Clinical Biochemistry, 30(1), 11–26. https://doi.org/10.1007/s12291-014-0446-0
Pieper, L., Meschede, T., Jung, M., Janowitz, U., & Schulze, M. (2023). Influence of equilibration time and bull-specific extender for cryopreservation on semen quality and fertility in german holstein friesian bulls: A controlled field trial. Animals, 13(14). Article 2285. https://doi.org/10.3390/ani13142285
Pintus, E., & Ros-Santaella, J. L. (2021). Impact of oxidative stress on male reproduction in domestic and wild animals. Antioxidants, 10(7), Article 1154. https://doi.org/10.3390/antiox10071154
Qamar, A. Y., Naveed, M. I., Raza, S., Fang, X., Roy, P. K., Bang, S., Tanga, B. M., Saadeldin, I. M., Lee, S., & Cho, J. (2023). Role of antioxidants in fertility preservation of sperm – A narrative review. Animal Bioscience, 36(3), 385–403. https://doi.org/10.5713/ab.22.0325
Raad, G., Lteif, L., Lahoud, R., Azoury, J., Azoury, J., Tanios, J., Hazzouri, M., & Azoury, J. (2018). Cryopreservation media differentially affect sperm motility, morphology and DNA integrity. Andrology, 6(6), 836–845. https://doi.org/10.1111/andr.12531
Raheja, N., Choudhary, S., Grewal, S., Sharma, N., & Kumar, N. (2018). A review on semen extenders and additives used in cattle and buffalo bull semen preservation. Journal of Entomology and Zoology Studies, 6(3), 239–245. https://www.entomoljournal.com/archives/?year=2018&vol=6&issue=3&ArticleId=3575
Roca, J., Martinez-Alborcia, M. J., Gil, M. A., Parrilla, I., & Martinez, E. A. (2013). Dead spermatozoa in raw semen samples impair in vitro fertilization outcomes of frozen-thawed spermatozoa. Fertility and Sterility, 100(3), 875–881. https://doi.org/10.1016/j.fertnstert.2013.05.020
Ruan, Q., Yang, S., Hua, S., Zhang, W., Li, D., Yang, Y., Wang, X., Wang, Q., & Meng, Z. (2024). Supplementation of extender with melatonin improves the motility, mitochondrial membrane potential, and fertilization ability of cryopreserved brown-marbled grouper sperm. Animals, 14(7), Article 995. https://doi.org/10.3390/ani14070995
Sabeti, P., Pourmasumi, S., Rahiminia, T., Akyash, F., & Reza Talebi, A. (2016). Etiologies of sperm oxidative stress. International Journal of Reproductive BioMedicine, 14(4), 231–240.
Saili, T., Nafiu, L. O., Pagala, M. A., Bain, A., Aku, A. S., Rahadi, S., Rusdin, M., & Lopulalan, F. (2023). Sperm quality of Bali bull following sexing and freezing using different cryoprotectants. IOP Conference Series: Earth and Environmental Science, 1241(1), Article 012137. https://doi.org/10.1088/1755-1315/1241/1/012137
Sakatani, M. (2022). [The role of reproductive biology in SDGs] Global warming and cattle reproduction: Will increase in cattle numbers progress to global warming? Journal of Reproduction and Development, 68(2), 90–95. https://doi.org/10.1262/jrd.2021-149
Sandfoss, M. R., Cantrell, J., Roberts, B. M., & Reichling, S. (2022). Cryopreservation of sperm from an endangered snake with tests of post-thaw incubation in caffeine. Animals, 12(14), Article 1824. https://doi.org/10.3390/ani12141824
Saranholi, D. A. C., De Paula, R. R., Pytilak, E., Afonso, F., Canela, L. F., De Almeida, A. B. M., Hidalgo, M. M. T., Martins, M. I. M., Blaschi, W., & Barreiros, T. R. R. (2021). Comparison of seminal characteristics of Aberdeen Angus, Holstein and Nelore bulls before and after cryopreservation. Research, Society and Development, 10(16), Article e408101623382. https://doi.org/10.33448/rsd-v10i16.23382
Sathe, S. (2021). Cryopreservation of semen. In M. Richard, D. V. M. Hopper, & A. C. T. Diplomat (Eds.), Bovine reproduction (pp. 986–999). John Wiley & Sons, Inc. https://doi.org/10.1002/9781119602484.ch78
Schieber, M., & Chandel, N. S. (2014). ROS function in redox signaling and oxidative stress. Current Biology, 24(10), 453–462. https://doi.org/10.1016/j.cub.2014.03.034
Schulze, M., Grobbel, M., Riesenbeck, A., Brüning, S., Schaefer, J., Jung, M., & Grossfeld, R. (2017). Dose rates of antimicrobial substances in boar semen preservation—time to establish new protocols. Reproduction in Domestic Animals, 52(3), 397–402. https://doi.org/10.1111/rda.12921
Seshoka, M. M., Mphaphathi, M. L., & Nedambale, T. L. (2016). Comparison of four different permitting and combination of two best cryoprotectants on freezing Nguni sperm evaluated with the aid of computer aided sperm analysis. Cryobiology, 72(3), 232–238. https://doi.org/10.1016/j.cryobiol.2016.04.001
Shah, S. A. H., Andrabi, S. M. H., & Qureshi, I. Z. (2016). Effect of equilibration times, freezing, and thawing rates on post-thaw quality of buffalo (Bubalus bubalis) bull spermatozoa. Andrology, 4(5), 972–976. https://doi.org/10.1111/andr.12214
Sharafi, M., Blondin, P., Vincent, P., Anzar, M., & Benson, J. D. (2022). Hydroxytyrosol and resveratrol improves kinetic and flow cytometric parameters of cryopreserved bull semen with low cryotolerance. Animal Reproduction Science, 245, Article 107065. https://doi.org/10.1016/j.anireprosci.2022.107065
Sharafi, M., Borghei-Rad, S. M., Hezavehei, M., Shahverdi, A., & Benson, J. D. (2022). Cryopreservation of semen in domestic animals: A review of current challenges, applications, and prospective strategies. Animals, 12(23), Article 3271. https://doi.org/10.3390/ani12233271
Sieme, H., & Oldenhof, H. (2015). Sperm cleanup and centrifugation processing for cryopreservation. In W. Wolkers & H. Oldenhof (Eds.), Cryopreservation and freeze-drying protocols (Vol. 1257, pp. 343-352). Springer. https://doi.org/10.1007/978-1-4939-2193-5_16
Silpa, M. V., König, S., Sejian, V., Malik, P. K., Nair, M. R. R., Fonseca, V. F. C., Maia, A. S. C., & Bhatta, R. (2021). Climate-resilient dairy cattle production: Applications of genomic tools and statistical models. Frontiers in Veterinary Science, 8, Article 625189. https://doi.org/10.3389/fvets.2021.625189
Silvestre, M. A., Yániz, J. L., Peña, F. J., Santolaria, P., & Castelló-Ruiz, M. (2021). Role of antioxidants in cooled liquid storage of mammal spermatozoa. Antioxidants, 10(7), Article 1096. https://doi.org/10.3390/antiox10071096
Simonik, O., Bubenickova, F., Tumova, L., Frolikova, M., Sur, V. P., Beran, J., Havlikova, K., Hackerova, L., Spevakova, D., Komrskova, K., & Postlerova, P. (2022). Boar sperm cryopreservation improvement using semen extender modification by dextran and pentaisomaltose. Animals, 12(7), Article 868. https://doi.org/10.3390/ani12070868
Slanina, T., Petrovičová, L., Miškeje, M., Kňížat, L., Mirda, J., Lukáč, N., Trandžík, J., Petrovičová, I., & Massányi, P. (2015). The effect of diluent, temperature and age on turkey spermatozoa motility in vitro. Journal of Applied Animal Research, 43(2), 131–136. https://doi.org/10.1080/09712119.2014.928627
Stuart, C. C., Vaughan, J. L., Kershaw, C. M., de Graaf, S. P., & Bathgate, R. (2019). Effect of diluent type, cryoprotectant concentration, storage method and freeze/thaw rates on the post-thaw quality and fertility of cryopreserved alpaca spermatozoa. Scientific Reports, 9, Article 12826. https://doi.org/10.1038/s41598-019-49203-z
Sztein, J. M., Takeo, T., & Nakagata, N. (2018). History of cryobiology, with special emphasis in evolution of mouse sperm cryopreservation. Cryobiology, 82, 57–63. https://doi.org/10.1016/j.cryobiol.2018.04.008
Thema, M. A., Mphaphathi, M. L., Ledwaba, M. R., & Nedambale, T. L. (2023). Sperm cryopreservation in Windsnyer boars; principles, technique, and updated outcomes. Animal Reproduction, 20(3), Article e20220100. https://doi.org/10.1590/1984-3143-AR2022-0100
Tvrdá, E., Massanyi, P., & Lukáč, N. (2018). Physiological and Pathological Roles of Free Radicals in Male Reproduction. In R. Meccariello, & R. Chianese (Eds.), Spermatozoa - Facts and Perspectives (pp. 117-157). InTech. https://doi.org/10.5772/intechopen.70793
Ugur, M. R., Saber Abdelrahman, A., Evans, H. C., Gilmore, A. A., Hitit, M., Arifiantini, R. I., Purwantara, B., Kaya, A., & Memili, E. (2019). Advances in Cryopreservation of Bull Sperm. Frontiers in Veterinary Science, 6, Article 268. https://doi.org/10.3389/fvets.2019.00268
Vanvanhossou, S. F. U., Dossa, L. H., & König, S. (2021). Sustainable management of animal genetic resources to improve low-input livestock production: Insights into local beninese cattle populations. Sustainability, 13(17), Article 9874. https://doi.org/10.3390/su13179874
Vázquez Gil, Á., & Guevara Viera, G. (2021). La genética molecular en la conservación de los recursos zoogenéticos. Revista de Producción Animal, 33(2), 83-101.
Vilela, C. G., Marquez, J. M., Graham, J. K., & Barfield, J. P. (2017). Cryopreservation of bison epididymal sperm: A strategy for improving post-thaw quality when collecting sperm in field conditions. Theriogenology, 89, 155–161. https://doi.org/10.1016/j.theriogenology.2016.09.044
Villaverde-Morcillo, S., Soler, A. J., Esteso, M. C., Castaño, C., Miñano-Berna, A., Gonzalez, F., & Santiago-Moreno, J. (2017). Immature and mature sperm morphometry in fresh and frozen-thawed falcon ejaculates. Theriogenology, 98, 94–100. https://doi.org/10.1016/j.theriogenology.2017.04.051
Víquez, L., Barquero, V., & Valverde, A. (2021). Optimal conditions for the kinematic analysis in fresh semen of Brahman bulls with a CASA-Mot system. Agronomía Mesoamericana, 32(3), 920–938. https://doi.org/10.15517/AM.V32I3.42768
Víquez, L., Sevilla, F., Araya-Zúñiga, I., Soler, C., Barquero, V., Roldan, E. R. S., & Valverde, A. (2023). Morphometric assessment of cryopreserved livestock bull spermatozoa in the tropics. Reproduction in Domestic Animals, 58(10), 1439–1447. https://doi.org/10.1111/rda.14459
Viudes de Castro, M. P., Talaván, A. G., & Vicente, J. S. (2021). Evaluation of dextran for rabbit sperm cryopreservation: Effect on frozen–thawed rabbit sperm quality variables and reproductive performance. Animal Reproduction Science, 226, Article 106714. https://doi.org/10.1016/j.anireprosci.2021.106714
Yánez-Ortiz, I., Catalán, J., Rodríguez-Gil, J. E., Miró, J., & Yeste, M. (2022). Advances in sperm cryopreservation in farm animals: Cattle, horse, pig and sheep. Animal Reproduction Science, 246, Article 106904. https://doi.org/10.1016/j.anireprosci.2021.106904
Yang, S. X., Adams, G. P., Palomino, J. M., Huanca, W. F., Lessard, C., Rajapaksha, K., & Anzar, M. (2020). Cryopreservation of bison semen without exogenous protein in extender and its fertility potential in vitro and in vivo following fixed-time artificial insemination. Theriogenology, 152, 156–164. https://doi.org/10.1016/j.theriogenology.2020.04.018
Zeitoun, M. M., & Al-Damegh, M. A. (2015). Effect of Nonenzymatic Antioxidants on Sperm Motility and Survival Relative to Free Radicals and Antioxidant Enzymes of Chilled-Stored Ram Semen. Open Journal of Animal Sciences, 5(1), 50–58. https://doi.org/10.4236/ojas.2015.51007
Archivos adicionales
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2025 Ignacio Araya-Zúñiga, Francisco Sevilla, José A. González, Kenneth Matamoros, Anthony Valverde

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
1. Política propuesta para revistas de acceso abierto
Los autores/as que publiquen en esta revista aceptan las siguientes condiciones:
- Los autores/as conservan los derechos morales de autor y ceden a la revista el derecho de la primera publicación, con el trabajo registrado con la licencia de atribución, no comercial y sin obra derivada de Creative Commons, que permite a terceros utilizar lo publicado siempre que mencionen la autoría del trabajo y a la primera publicación en esta revista, no se puede hacer uso de la obra con propósitos comerciales y no se puede utilizar las publicaciones para remezclar, transformar o crear otra obra.
- Los autores/as pueden realizar otros acuerdos contractuales independientes y adicionales para la distribución no exclusiva de la versión del artículo publicado en esta revista (p. ej., incluirlo en un repositorio institucional o publicarlo en un libro) siempre que indiquen claramente que el trabajo se publicó por primera vez en esta revista.
- Se permite y recomienda a los autores/as a publicar su trabajo en Internet (por ejemplo en páginas institucionales o personales) antes y durante el proceso de revisión y publicación, ya que puede conducir a intercambios productivos y a una mayor y más rápida difusión del trabajo publicado (vea The Effect of Open Access).