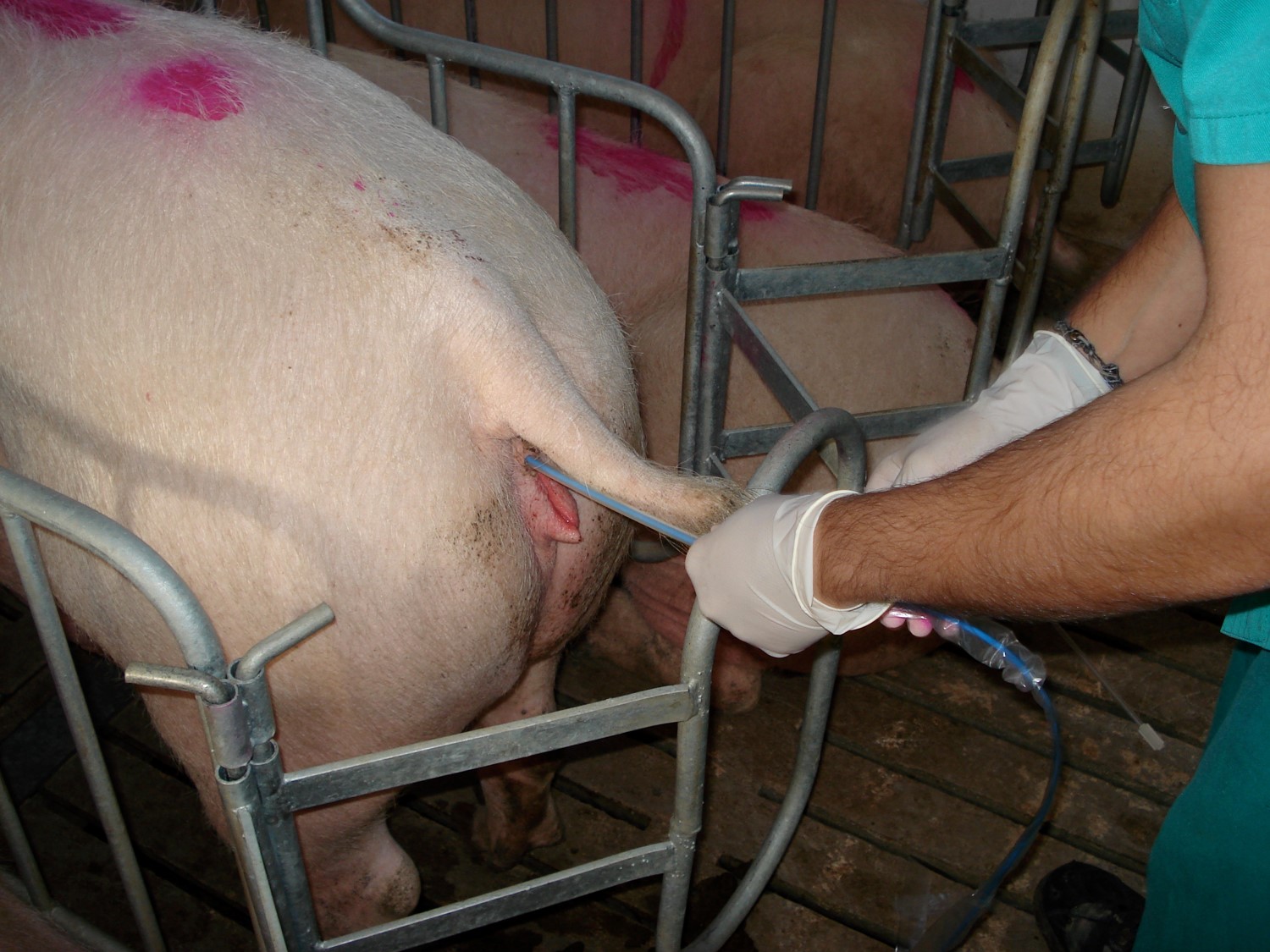
Biotecnología aplicada al estudio de la movilidad del semen porcino
DOI:
https://doi.org/10.15517/am.v32i2.40628Palabras clave:
eyaculado, reproducción, verraco, inseminación artificialResumen
Introducción. El uso de biotecnologías reproductivas ha significado una mejora relevante sobre los parámetros de eficiencia y rentabilidad en la industria porcina. Objetivo. Determinar el estado del arte en el que se encuentra la biotecnología aplicada al estudio del semen y la implementación; en granjas porcinas; de técnicas de reproducción asistida como la inseminación artificial. Desarrollo. En el sector porcino, la fertilidad de los verracos (Sus scrofa domestica) tiene un efecto significativo sobre la eficiencia general de la reproducción de las piaras. Aunque hay métodos objetivos de valoración precisa de las variables seminales de los verracos, muchas granjas aún continúan realizando sus evaluaciones de forma subjetiva. Los conceptos bioquímicos que determinan la funcionalidad del gameto masculino o espermatozoide son molecularmente complejos, sin embargo, pueden conocerse los aspectos generales de la funcionalidad y desarrollar métodos de manejo del semen que permita el mantenimiento de las condiciones óptimas de las muestras seminales o dosis de semen utilizadas en inseminación artificial (IA). Dentro de la funcionalidad espermática intervienen parámetros clásicos de volumen, concentración y movilidad que son fundamentales para la optimización de las dosis seminales producidas por verraco. Los sistemas CASA (Computerassisted semen analysis) son una aplicación biotecnológica que desplaza el análisis subjetivo tradicional del semen para la valoración de la calidad, estos aportan grandes volúmenes de información que han permitido explorar más en detalle los eyaculados porcinos. Conclusiones. En las granjas que utilizan la inseminación artificial, el uso de técnicas biotecnológicas para el análisis seminal, constituye un valor agregado para el estudio de la calidad del semen y la optimización de dosis seminales producidas.
Descargas
Citas
Althouse, G., Kuster, C., Clark, S., & Weisiger, R. (2000). Field investigations of bacterial contaminants and their effects on extended porcine semen. Theriogenology, 53(5), 1167–1176. https://doi.org/10.1016/S0093-691X(00)00261-2
Althouse, G., & Lu, K. G. (2005). Bacteriospermia in extended porcine semen. Theriogenology, 63(2 SPEC. ISS.), 573–584. https://doi.org/10.1016/j.theriogenology.2004.09.031
Amann, R., & Katz, D. F. (2004). Andrology Lab Corner*: Reflections on CASA after 25 years. Journal of Andrology, 25(3), 317–325. https://doi.org/10.1002/j.1939-4640.2004.tb02793.x
Amann, R., & Waberski, D. (2014). Computer-assisted sperm analysis (CASA): Capabilities and potential developments. Theriogenology, 81(1), 5–17.e3. https://doi.org/10.1016/J.THERIOGENOLOGY.2013.09.004
Aneas, S. B., Gary, B. G., & Bouvier, B. P. (2008). Collectis® automated boar collection technology. Theriogenology, 70(8), 1368–1373. https://doi.org/10.1016/j.theriogenology.2008.07.011
Audet, I., Laforest, J. P., Martineau, G. P., & Matte, J. J. (2004). Effect of vitamin supplements on some aspects of performance, vitamin status, and semen quality in boars. Journal of Animal Science, 82(2), 626–633. https://doi.org/10.2527/2004.822626x
Awda, B. J., & Buhr, M. M. (2008). The effect of glove type on boar semen quality. Theriogenology, 70(8), 1388. https://doi.org/10.1016/j.theriogenology.2008.06.046
Ax, R. L., Dally, M., Didion, B. A., Lenz, R. W., Love, C. C., Varner, D. D., Hafez, B., & Bellin, M. E. (2016). Semen evaluation. In B. Hafez & E. Hafez (Eds.), Reproduction in farm animals (pp. 363–375). Lippincott Williams & Wilkins. https://doi.org/10.1002/9781119265306.ch25
Banaszewska, D., & Kondracki, S. (2012). An assessment of the breeding maturity of insemination boars based on ejaculate quality changes. Folia Biologica, 60(3), 151–162. https://doi.org/10.3409/fb60_34.151162
Björndahl, L., & Kvist, U. (2009). Human sperm chromatin stabilization: A proposed model including zinc bridges. Molecular Human Reproduction, 16(1), pp. 23–29. https://doi.org/10.1093/molehr/gap099
Bompart, D., García-Molina, A., Valverde, A., Caldeira, C., Yániz, J., Núñez de Murga, M., & Soler, C. (2018). CASAMot technology: how results are affected by the frame rate and counting chamber. Reproduction, Fertility and Development, 30(6), 810–819. https://doi.org/10.1071/RD17551
Bonet, S., Casas, I., Holt, W., & Yeste, M. (2013). Boar reproduction : fundamentals and new biotechnological trends. In S. Bonet, I. Casas, W. V. Holt, & M. Yeste (Eds.), Boar Reproduction (1st ed., p. 632). Springer-Verlag Berlin Heidelberg.
Boryshpolets, S., Kowalski, R. K., Dietrich, G. J., Dzyuba, B., & Ciereszko, A. (2013). Different computer assisted sperm analysis (CASA) systems highly influence sperm motility parameters. Theriogenology, 80(7), 758–765. https://doi.org/10.1016/j.theriogenology.2013.06.019
Brinsko, S. P., Blanchard, T. L., Varner, D. D., Schumacher, J., Love, C. C., Hinrichs, K., & Hartman, D. L. (2011). Examination of the stallion for breeding soundness. In S. P. Brinsko, T. L. Blanchard, D. D. Varner, J. Schumacher, & C. C. Love (Eds.), Manual of Equine Reproduction (pp. 176–206). Elsevier. https://doi.org/10.1016/b978-0-323-06482-8.00022-3
Britt, J., Almond, G., & Flowers, W. (1999). Diseases of the reproductive system. In B. Straw, S. D’Allaire, W. Mengeling, & D. Taylor (Eds.), Diseases of Swine (8th Ed., p. 905). Blackwell Science Ltd.
Broekhuijse, M. L. W. J., Šoštarić, E., Feitsma, H., & Gadella, B. M. (2012). Application of computer-assisted semen analysis to explain variations in pig fertility. Journal of Animal Science, 90(3), 779–789. https://doi.org/10.2527/jas.2011-4311
Buhr, M. M., Fiser, P., Bailey, J. L., & Curtis, E. F. (2001). Cryopreservation in different concentrations of glycerol alters boar sperm and their membranes. Journal of Andrology, 22(6), 961–969. https://doi.org/10.1002/j.1939-4640.2001.tb03436.x
Castellini, C., Dal Bosco, A., Ruggeri, S., & Collodel, G. (2011). What is the best frame rate for evaluation of sperm motility in different species by computer-assisted sperm analysis? Fertility and Sterility, 96(1), 24–27. https://doi.org/10.1016/j.fertnstert.2011.04.096
Clemente-Sánchez, F., Cessa-Reyes, V., Cortez-Romero, C., Tarango-Arambula, L. A., & Arenas-Baez, P. (2015). Commercial extenders and freezing curves for the preservation of sperm cells of white-tailed deer (Odocoileus virginianus). Journal of Applied Animal Research, 43(4), 468–473. https://doi.org/10.1080/09712119.2014.980422
Clements, K. M., Shipley, C. F., Coleman, D. A., Ehrhart, E. J., Haschek, W. M., & Clark, S. G. (2010). Azoospermia in an 8-month-old boar due to bilateral obstruction at the testis/epididymis interface. The Canadian Veterinary Journal = La Revue Veterinaire Canadienne, 51(10), 1130–1134. http://www.ncbi.nlm.nih.gov/pubmed/21197205
Colagar, A. H., Marzony, E. T., & Chaichi, M. J. (2009). Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutrition Research, 29(2), 82–88. https://doi.org/10.1016/j.nutres.2008.11.007
Cummins, J. (2009). Sperm motility and energetics. In T. Birkhead, D. Hosken, & S. Pitnick (Eds.), Sperm biology, An evolutionary perspective (pp. 185–206). Academic Press.
Davies, D. C., Hall, G., Hibbitt, K. G., & Moore, H. D. M. (1975). The removal of the seminal vesicles from the boar and the effects on the semen characteristics. Journal of Reproduction and Fertility, 43(2), 305–312. https://doi.org/10.1530/jrf.0.0430305
de Catanzaro, D., & Pollock, T. (2016). Absorption and distribution of estradiol from male seminal emissions during mating. Journal of Endocrinology, 231(3), 245–257. https://doi.org/10.1530/JOE-16-0247
Ehlers, J., Behr, M., Bollwein, H., Beyerbach, M., & Waberski, D. (2011). Standardization of computer-assisted semen analysis using an e-learning application. Theriogenology, 76(3), 448–454. https://doi.org/10.1016/j.theriogenology.2011.02.021
Estienne, M. J., & Harper, A. F. (2004). Semen characteristics and libido in boars treated repeatedly with PGF 2α. Journal of Animal Science, 82(5), 1494–1498. https://doi.org/10.2527/2004.8251494x
Flowers, W. (2015). Factors affecting the efficient production of boar sperm. Reproduction in Domestic Animals, 50, 25–30. https://doi.org/10.1111/rda.12529
Flowers, W. L. (1997). Management of boars for efficient semen production. Journal of Reproduction and Fertility. Supplement, 52, 67–78. http://www.ncbi.nlm.nih.gov/pubmed/9602720
Frangež, R., Gider, T., & Kosec, M. (2005). Frequency of boar ejaculate collection and its influence on semen quality, pregnancy rate and litter size. Acta Veterinaria (Brno), 74(2), 265–273. https://doi.org/10.2754/avb200574020265
Gallagher, M. T., Smith, D. J., & Kirkman-Brown, J. C. (2018). CASA: tracking the past and plotting the future. Reproduction, Fertility and Development, 30(6), 867–874. https://doi.org/10.1071/RD17420
García-Herreros, M. (2016). Sperm subpopulations in avian species: a comparative study between the rooster (Gallus domesticus) and Guinea fowl (Numida meleagris). Asian Journal of Andrology, 18(6), 889 894. https://doi.org/10.4103/1008-682X.188448
García-Molina, A., Valverde, A., Bompart, D., Caldeira, C., Vendrell, A., & Soler, C. (2019). Updating semen analysis: a subpopulation approach. Asian Journal of Andrology, 22(1), 118–119. https://doi.org/10.4103/aja.aja_33_19
Gogol, P., Szczesniak-Fabiańczyk, B., & Wierzchoś-Hilczer, A. (2009). The photon emission, ATP level and motility of boar spermatozoa during liquid storage. Reproductive Biology, 9(1), 39–49. https://doi.org/10.1016/S1642-431X(12)60093-X
Goldberg, A., Argenti, L. E., Faccin, J. E., Linck, L., Santi, M., Lourdes Bernardi, M., Cardoso, M. R., Wentz, I., & Bortolozzo, F. P. (2013). Risk factors for bacterial contamination during boar semen collection. Research in Veterinary Science, 95, 362–367. https://doi.org/10.1016/j.rvsc.2013.06.022
Guillette, J. L. J., Brock, J. W., Rooney, A. A., & Woodward, A. R. (1999). Serum concentrations of various environmental contaminants and their relationship to sex steroid concentrations and phallus size in juvenile american alligators. Archives of Environmental Contamination and Toxicology, 36(4), 447–455. https://doi.org/10.1007/PL00006617
Guthrie, H. D., Welch, G. R., & Long, J. A. (2008). Mitochondrial function and reactive oxygen species action in relation to boar motility. Theriogenology, 70(8), 1209–1215. https://doi.org/10.1016/j.theriogenology.2008.06.017
Herrick, J. (1950). Artificial insemination of swine. Iowa State University Veterinarian. https://lib.dr.iastate.edu/iowastate_veterinarian/vol12/iss1/4
Hess, K. C., Jones, B. H., Marquez, B., Chen, Y., Ord, T. S., Kamenetsky, M., Miyamoto, C., Zippin, J. H., Kopf, G. S., Suarez, S. S., Levin, L. R., Williams, C. J., Buck, J., & Moss, S. B. (2005). The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Developmental Cell, 9(2), 249–259. https://doi.org/10.1016/j.devcel.2005.06.007
Houška, L., Wolfová, M., & Fiedler, J. (2004). Economic weights for production and reproduction traits of pigs in the Czech Republic. Livestock Production Science, 85(2–3), 209–221. https://doi.org/10.1016/S0301-6226(03)00128-3
Jiménez-Rabadán, P., Ramón, M., García-Álvarez, O., Maroto-Morales, A., del Olmo, E., Pérez-Guzmán, M. D., Bisbal, A., Fernández-Santos, M. R., Garde, J. J., & Soler, A. (2012). Effect of semen collection method (artificial vagina vs. electroejaculation), extender and centrifugation on post-thaw sperm quality of Blanca-Celtibérica buck ejaculates. Animal Reproduction Science, 132(1–2), 88–95. https://doi.org/10.1016/j.anireprosci.2012.04.005
Johnson, L. A., Weitze, K. F., Fiser, P., & Maxwell, W. M. (2000). Storage of boar semen. Animal Reproduction Science, 62(1–3), 143–172. http://www.ncbi.nlm.nih.gov/pubmed/10924823
Jones, A. R., & Bubb, W. A. (2000). Substrates for endogenous metabolism by mature boar spermatozoa. Journal of Reproduction and Fertility, 119(1), 129–135. https://doi.org/10.1530/reprod/119.1.129
Kaysen, B. L., Levalley, S. B., Ames, D. R., Dalsted, N. L., Schwab, C. R., Daryl, J., Cleon, T., & Kimberling, V. (2013). Dissertation factors that impact probability of pregnancy when using ai boars. Colorado State University.
Knecht, D., Jankowska-Mąkosa, A., & Duziński, K. (2017). The effect of age, interval collection and season on selected semen parameters and prediction of AI boars productivity. Livestock Science, 201, 13–21. https://doi.org/10.1016/j.livsci.2017.04.013
Knecht, D., Środoń, S., Szulc, K., & Duziński, K. (2013). The effect of photoperiod on selected parameters of boar semen. Livestock Science, 157(1), 364–371. https://doi.org/10.1016/j.livsci.2013.06.027
Knox, R. V. (2016). Artificial insemination in pigs today. Theriogenology, 85(1), 83–93. https://doi.org/10.1016/j.theriogenology.2015.07.009
Ko, J. C. H., Evans, L. E., & Althouse, G. (1989). Toxicity effects of latex gloves on boar spermatozoa. Theriogenology, 31(6), 1159–1164. https://doi.org/10.1016/0093-691X(89)90084-8
Kodentsova, V. M., Vrzhesinskaya, O. A., & Evdokimov, V. V. (2003). Riboflavin content in male spermoplasm. Bulletin of Experimental Biology and Medicine, 135(3), 258–260. https://doi.org/10.1023/A:1024184914560
Kondracki, S., Iwanina, M., Wysokińska, A., & Huszno, M. (2012). Comparative analysis of Duroc and Pietrain boar sperm morphology. Acta Veterinaria Brno, 81(2), 195–199. https://doi.org/10.2754/avb201281020195
Krause, W. (1995). Computer-assisted semen analysis systems: comparison with routine evaluation and prognostic value in male fertility and assisted reproduction. Human Reproduction, 10(Suppl. 1), 60–66. http://www.ncbi.nlm.nih.gov/pubmed/8592042
Kumaresan, A., Kadirvel, G., Bujarbaruah, K. M., Bardoloi, R. K., Das, A., Kumar, S., & Naskar, S. (2009). Preservation of boar semen at 18 °C induces lipid peroxidation and apoptosis like changes in spermatozoa. Animal Reproduction Science, 110(1–2), 162–171. https://doi.org/10.1016/j.anireprosci.2008.01.006
Kuster, C. (2005). Sperm concentration determination between hemacytometric and CASA systems: Why they can be different. Theriogenology, 64(3), 614–617. https://doi.org/10.1016/J.THERIOGENOLOGY.2005.05.047
Lavara, R., Vicente, J. S., & Baselga, M. (2013). Genetic variation in head morphometry of rabbit sperm. Theriogenology, 80(4), 313–318. https://doi.org/10.1016/J.THERIOGENOLOGY.2013.04.015
Ledesma, A., Zalazar, L., Fernández-Alegre, E., Hozbor, F., Cesari, A., & Martínez-Pastor, F. (2017). Seminal plasma proteins modify the distribution of sperm subpopulations in cryopreserved semen of rams with lesser fertility. Animal Reproduction Science, 184, 44–50. https://doi.org/10.1016/j.anireprosci.2017.06.015
Lelono, A., Riedstra, B., & Groothuis, T. (2019). Ejaculate testosterone levels affect maternal investment in red junglefowl (Gallus gallus gallus). Scientific Reports, 9(1), Article 12126. https://doi.org/10.1038/s41598-019-48563-w
Leonhard-Marek, S. (2001). Influence of drugs, pollution and trace elements on male fertility. In W. Busch, & A. Holzmann (Eds.), Andrology in veterinary medicine (pp. 474–481). Schattauer.
Levis, D. G., & Reicks, D. L. (2005). Assessment of sexual behavior and effect of semen collection pen design and sexual stimulation of boars on behavior and sperm output - A review. Theriogenology, 63(2 SPEC. ISS.), 630–642. https://doi.org/10.1016/j.theriogenology.2004.09.037
Li, X., Wang, L., Li, Y., Zhao, N., Zhen, L., Fu, J., & Yang, Q. (2016). Calcium regulates motility and protein phosphorylation by changing cAMP and ATP concentrations in boar sperm in vitro. Animal Reproduction Science, 172, 39–51. https://doi.org/10.1016/j.anireprosci.2016.07.001
Lopez-Rodriguez, A., Soom, A. Van, Arsenakis, I., & Maes, D. (2017). Boar management and semen handling factors affect the quality of boar extended semen. Porcine Health Management, 3, Article 15. https://doi.org/10.1186/s40813-017-0062-5
López Rodríguez, A., Rijsselaere, T., Beek, J., Vyt, P., Van Soom, A., & Maes, D. (2013). Boar seminal plasma components and their relation with semen quality. Systems Biology in Reproductive Medicine, 59(1), 5–12. https://doi.org/10.3109/19396368.2012.725120
Maes, D., López Rodríguez, Alfonso Rijsselaere, T., Vyt, P., & Van Soom, A. (2011). Artificial Insemination in Pigs. In E. Manafi (Ed.), Artificial insemination in farm animals (pp. 79–94). In Tech.
Marin-Guzman, J., Mahan, D. C., & Whitmoyer, R. (2000). Effect of dietary selenium and vitamin E on the ultrastructure and ATP concentration of boar spermatozoa, and the efficacy of added sodium selenite in extended semen on sperm motility. Journal of Animal Science, 78(6), 1544–1550. https://doi.org/10.2527/2000.7861544x
Maroto-Martín, L. O., Muñoz, E. C., De Cupere, F., Van Driessche, E., Echemendia-Blanco, D., Rodríguez, J. M. M., & Beeckmans, S. (2010). Bacterial contamination of boar semen affects the litter size. Animal Reproduction Science, 120(1–4), 95–104. https://doi.org/10.1016/j.anireprosci.2010.03.008
Martinez-Alborcia, M. J., Valverde, A., Parrilla, I., Vazquez, J. M., Martinez, E. A., & Roca, J. (2012). Detrimental effects of non-functional spermatozoa on the freezability of functional spermatozoa from Boar Ejaculate. PLoS ONE, 7(5), Article e36550. https://doi.org/10.1371/journal.pone.0036550
Massányi, P., Trandzik, J., Nad, P., Toman, R., Skalická, M., & Koréneková, B. (2003). Seminal concentrations of trace elements in various animals and their correlations. Asian Journal of Andrology, 5(2), 101–104. http://www.ncbi.nlm.nih.gov/pubmed/12778318
McPherson, F. J., Nielsen, S. G., & Chenoweth, P. J. (2014). Semen effects on insemination outcomes in sows. Animal Reproduction Science, 151(1–2), 28–33. https://doi.org/10.1016/J.ANIREPROSCI.2014.09.021
Medrano, A., Fernández-Novell, J. M., Ramió, L., Alvarez, J., Goldberg, E., Rivera, M. M., Guinovart, J. J., Rigau, T., & Rodríguez-Gil, J. E. (2006). Utilization of citrate and lactate through a lactate dehydrogenase and ATP regulated pathway in boar spermatozoa. Molecular Reproduction and Development, 73(3), 369–378. https://doi.org/10.1002/mrd.20414
Mortimer, S. T., Van Der Horst, G., & Mortimer, D. (2015). The future of computer-aided sperm analysis. Asian Journal of Andrology, 17(4), 545–553. https://doi.org/10.4103/1008-682X.154312
Murase, T., Imaeda, N., Yamada, H., & Miyazawa, K. (2007). Seasonal changes in semen characteristics, composition of seminal plasma and frequency of acrosome reaction induced by calcium and calcium ionophore A23187 in large white boars. Journal of Reproduction and Development, 53(4), 853–865. https://doi.org/10.1262/jrd.19026
Naughton, C. K., Nelson, D. R., & Thomas, A. J. (2003). Development of an Inexpensive Artificial Vagina for Semen Collection from Rabbits. Journal of Andrology, 24(5), 712–715. https://doi.org/10.1002/j.1939-4640.2003.tb02731.x
Okamura, N., Tajima, Y., Soejima, A., Masuda, H., & Sugita, Y. (1985). Sodium bicarbonate in seminal plasma stimulates the motility of mammalian spermatozoa through direct activation of adenylate cyclase. The Journal of Biological Chemistry, 260(17), 9699–9705. http://www.ncbi.nlm.nih.gov/pubmed/2991260
Park, S. (2013). Effects of sow, boar, and semen traits on sow reproduction. University of Nebraska-Lincoln. https://digitalcommons.unl.edu/animalscidiss/67
Pesch, S., Bergmann, M., & Bostedt, H. (2006). Determination of some enzymes and macro- and microelements in stallion seminal plasma and their correlations to semen quality. Theriogenology, 66(2), 307–313. https://doi.org/10.1016/j.theriogenology.2005.11.015
Pieczyńska, J., & Grajeta, H. (2015). The role of selenium in human conception and pregnancy. Journal of Trace Elements in Medicine and Biology, 29, 31–38. https://doi.org/10.1016/j.jtemb.2014.07.003
Pipan, M. Z., Mrkun, J., Strajn, B. J., Vrtač, K. P., Kos, J., Pišlar, A., & Zrimšek, P. (2017). The influence of macro-and microelements in seminal plasma on diluted boar sperm quality. Acta Veterinaria Scandinavica, 59(1), Article 11. https://doi.org/10.1186/s13028-017-0279-y
Pruneda, A., Pinart, E., Briz, M. D., Sancho, S., Garcia-Gil, N., Badia, E., Kadar, E., Bassols, J., Bussalleu, E., Yeste, M., & Bonet., S. (2005). Effects of a high semen-collection frequency on the quality of sperm from ejaculates and from six epididymal regions in boars. Theriogenology, 63, 2219–2232. https://doi.org/10.1016/j.theriogenology.2004.10.009
Quirós-Rojas, M., Madrigal-Valverde, M., Camacho-Calvo, M., & Valverde, A. (2018). Efecto del catéter de inseminación y el orden de parto sobre parámetros de producción en ganado porcino. Revista Tecnología En Marcha, 31(3), 86–97. https://doi.org/10.18845/tm.v31i3.3905
Ramió, L., Rivera, M. M., Ramírez, A., Concha, I. I., Peña, A., Rigau, T., & Rodríguez-Gil, J. E. (2008). Dynamics of motilesperm subpopulation structure in boar ejaculates subjected to “in vitro” capacitation and further “in vitro” acrosome reaction. Theriogenology, 69(4), 501–512. https://doi.org/10.1016/j.theriogenology.2007.10.021
Ramón, M., & Martínez-Pastor, F. (2018). Implementation of novel statistical procedures and other advanced approaches to improve analysis of CASA data. Reproduction, Fertility and Development, 30(6), 860-866. https://doi.org/10.1071/RD17479
Ratchamak, R., Vongpralub, T., Boonkum, W., & Chankitisakul, V. (2019). Cryopreservation and quality assessment of boar semen collected from bulk samples. Veterinarni Medicina, 64(05), 209–216. https://doi.org/10.17221/125/2018-VETMED
Ribeiro, J. C., Carvalho, L. E., Sousa, K. C., & Nepomuceno, R. C. (2008). Prolificidade de fêmeas suínas na cidade de Fortaleza, Ceará, Brasil. Archivos de Zootecnia, 57(220), 537–540. http://www.redalyc.org/articulo.oa?id=49515034015
Robinson, J. A. B., & Buhr, M. M. (2005). Impact of genetic selection on management of boar replacement. Theriogenology, 63(2), 668–678. https://doi.org/10.1016/j.theriogenology.2004.09.040
Rodríguez-Gil, J. E., & Bonet, S. (2016). Current knowledge on boar sperm metabolism: Comparison with other mammalian species. Theriogenology, 85(1), 4–11. https://doi.org/10.1016/j.theriogenology.2015.05.005
Rodríguez-Martínez, H., Kvist, U., Ernerudh, J., Sanz, L., & Calvete, J. J. (2011). Seminal plasma proteins: What role do they play? American Journal of Reproductive Immunology, 66(SUPPL. 1), 11–22. https://doi.org/10.1111/j.1600-0897.2011.01033.x
Rozeboom, K. (2000). Evaluating Boar Semen Quality (ANS 00-812S). North Carolina State University. https://projects.ncsu.edu/project/swine_extension/publications/factsheets/812s.htm
Sağlam, H. S., Altundağ, H., Atik, Y. T., Dündar, M. Ş., & Adsan, Ö. (2015). Trace elements levels in the serum,urine, and semen of patients with infertility. Turkish Journal of Medical Sciences, 45(2), 443–448. https://doi.org/10.3906/sag-1402-140
Seligman, J., Newton, G. L., Fahey, R. C., Shalgi, R., & Kosower, N. S. (2005). Nonprotein thiols and disulfides in rat epididymal spermatozoa and epididymal fluid: Role of γ-glutamyl-transpeptidase in sperm maturation. Journal of Andrology, 26(5), 629–637. https://doi.org/10.2164/jandrol.05040
Sepúlveda, L., Bussalleu, E., Yeste, M., & Bonet, S. (2014). Effects of different concentrations of Pseudomonas aeruginosa on boar sperm quality. Animal Reproduction Science, 150(3–4), 96–106. https://doi.org/10.1016/j.anireprosci.2014.09.001
Smital, J., Sousa, L. L. De, & Mohsen, A. (2004). Differences among breeds and manifestation of heterosis in AI boar sperm output. Animal Reproduction Science, 80, 121–130. https://doi.org/10.1016/S0378-4320(03)00142-8
Smith, S. C., & England, G. C. (2001). Effect of technical settings and semen handling upon motility characteristics of dog spermatozoa measured using computer-aided sperm analysis. Journal of Reproduction and Fertility. Supplement, 57, 151–159. http://www.ncbi.nlm.nih.gov/pubmed/11787144
Soler, C., Perez-Sanchez, F., Schulze, H., Bergmann, M., Oberpenning, F., Yeung, C.-H., & Cooper, T. (2000). Objective evaluation of the morphology of human epididymal sperm heads. International Journal of Andrology, 23(2), 77–84. https://doi.org/10.1046/j.1365-2605.2000.00211.x
Soler, C., Valverde, A., Bompart, D., Fereidounfar, S., Sancho, M., Yániz, J., Garcia-Molina, A., & Korneenko-Zhilyaev, Y. (2017). New methods of semen analysis by casa. Sel’skokhozyaistvennaya Biologiya (Agricultural Biology), 52(2), 232–241. https://doi.org/10.15389/agrobiology.2017.2.232eng
Strzežek, J., Korda, W., Glogowski, J., Wysocki, P., & Borkowski, K. (1995). Influence of Semen-collection Frequency on Sperm Quality in Boars, with Special Reference to Biochemical Markers. Reproduction in Domestic Animals, 30(2), 85–94. https://doi.org/10.1111/j.1439-0531.1995.tb00609.x
Surai, P. F., & Fisinin, V. I. (2015). Selenium in pig nutrition and reproduction: Boars and semen quality - A review. Asian-Australasian Journal of Animal Sciences, 28(5), 730–736. https://doi.org/10.5713/ajas.14.0593
Tejerina, F., Buranaamnuay, K., Saravia, F., Wallgren, M., & Rodriguez-Martinez, H. (2008). Assessment of motility of ejaculated, liquid-stored boar spermatozoa using computerized instruments. Theriogenology, 69(9), 1129–1138. https://doi.org/10.1016/j.theriogenology.2008.01.027
Töpfer-Petersen, E., Romero, A., Varela, P. F., Ekhlasi-Hundrieser, M., Dostàlovà, Z., Sanz, L., & Calvete, J. J. (2009). Spermadhesins: A new protein family. Facts, hypotheses and perspectives. Andrologia, 30(4–5), 217–224. https://doi.org/10.1111/j.1439-0272.1998.tb01163.x
Tosky, E., Dysart, N., Swing, S., & Flowers, W. (2013). Libido, semen characteristics and fertility of boars housed in crates versus pens. Journal of Animal Science, 91(Suppl 2), 123.
Tourmente, M., & Roldan, E. R. S. (2015). Mass-specific metabolic rate influences sperm performance through energy production in mammals. PLoS ONE, 10(9), Article e0138185. https://doi.org/10.1371/journal.pone.0138185
Tremoen, N. H., Gaustad, A. H., Andersen-Ranberg, I., van Son, M., Zeremichael, T. T., Frydenlund, K., Grindflek, E., Våge, D. I., & Myromslien, F. D. (2018). Relationship between sperm motility characteristics and ATP concentrations, and association with fertility in two different pig breeds. Animal Reproduction Science, 193, 226–234. https://doi.org/10.1016/J.ANIREPROSCI.2018.04.075
Tsujii, H., Ohta, E., Miah, A. G., Hossain, S., & Salma, U. (2006). Effect of fructose on motility, acrosome reaction and in vitro fertilization capability of boar spermatozoa. Reproductive Medicine and Biology, 5(4), 255–261. https://doi.org/10.1111/j.1447-0578.2006.00150.x
Turner, R. M. (2003). Tales from the Tail: What Do We Really Know about Sperm Motility? Journal of Andrology, 24(6), 790–803. https://doi.org/10.1002/j.1939-4640.2003.tb03123.x
Valverde, A., Areán, H., Fernández, A., Bompart, D., García-Molina, A., López-Viana, J., & Soler, C. (2019a). Combined effect of type and capture area of counting chamber and diluent on Holstein bull sperm kinematics. Andrologia, 51(4), e13223. https://doi.org/10.1111/and.13223
Valverde, A., Arenán, H., Sancho, M., Contell, J., Yániz, J., Fernández, A., & Soler, C. (2016). Morphometry and subpopulation structure of Holstein bull spermatozoa: variations in ejaculates and cryopreservation straws. Asian Journal of Andrology, 18(6), 851–857. https://doi.org/10.4103/1008-682X.187579
Valverde, A., Castro-Morales, O., Madrigal-Valverde, M., & Soler, C. (2019b). Sperm kinematics and morphometric subpopulations analysis with CASA systems: A review. Revista de Biologia Tropical, 67(6), 1473–1487. https://doi.org/10.15517/rbt.v67i6.35151
Valverde, A., & Madrigal-Valverde, M. (2019). Evaluación de cámaras de recuento sobre parámetros espermáticos de verracos analizados con un sistema CASA-Mot. Agronomía Mesoamericana, 30(2), 447–458. https://doi.org/10.15517/am.v30i1.34145
Valverde, A., Madrigal-Valverde, M., Caldeira, C., Bompart, D., Núñez de Murga, J., Arnau, S., & Soler, C. (2019c). Effect of frame rate capture frequency on sperm kinematic parameters and subpopulation structure definition in boars, analyzed with a CASA-Mot system. Reproduction in Domestic Animals, 54(2), 167–175. https://doi.org/10.1111/rda.13320
Valverde, A., Madrigal-Valverde, M., Castro-Morales, O., Gadea-Rivas, A., Johnston, S., & Soler, C. (2019d). Kinematic and head morphometric characterisation of spermatozoa from the Brown Caiman (Caiman crocodilus fuscus). Animal Reproduction Science, 207, 9–20. https://doi.org/10.1016/J.ANIREPROSCI.2019.06.011
Valverde, A., Madrigal-Valverde, M., Lotz, J., Bompart, D., & Soler, C. (2019e). Effect of video capture time on sperm kinematic parameters in breeding boars. Livestock Science, 220, 52–56. https://doi.org/10.1016/j.livsci.2018.12.008
Valverde, A., Madrigal-Valverde, M., Solís-Arias, J., & Paniagua-Madrigal, W. (2019f). Variabilidad en los métodos de estimación de la concentración espermática en verracos. Agronomía Costarricense, 43(2), 25–43. https://doi.org/10.15517/rac.v43i2.37793
van der Horst, G., Maree, L., & du Plessis, S. (2018). Current perspectives of CASA applications in diverse mammalian spermatozoa. Reproduction, Fertility and Development, 30(6), 875–888. https://doi.org/10.1071/RD17468
Verstegen, J., Iguer-Ouada, M., & Onclin, K. (2002). Computer assisted semen analyzers in andrology research and veterinary practice. Theriogenology, 57(1), 149–179. https://doi.org/10.1016/S0093-691X(01)00664-1
Víquez, L., Barquero, V., Soler, C., Roldan, E. R. S., & Valverde, A. (2020). Kinematic sub-populations in bull spermatozoa: A Comparison of Classical and Bayesian Approaches. Biology, 9(6), Article 138. https://doi.org/10.3390/biology9060138
Waberski, D., Petrunkina, A. M., & Töpfer-Petersen, E. (2008). Can external quality control improve pig AI efficiency? Theriogenology, 70(8), 1346–1351. https://doi.org/10.1016/j.theriogenology.2008.06.006
World Health Organization. (2016). WHO laboratory manual for the examination and processing of human semen. https://www.who.int/publications/i/item/9789241547789
Wu, A. S. H., Oldfield, J. E., Shull, L. R., & Cheeke, P. R. (1979). Specific Effect of Selenium Deficiency on Rat Sperm1. Biology of Reproduction, 20(4), 793–798. https://doi.org/10.1095/biolreprod20.4.793
Yániz, J., Silvestre, M., Santolaria, P., & Soler, C. (2018). CASA-Mot in mammals: an update. Reproduction, Fertility, and Development, 30(6), 799–809. https://doi.org/10.1071/RD17432
Archivos adicionales
Publicado
Cómo citar
Número
Sección
Licencia
1. Política propuesta para revistas de acceso abierto
Los autores/as que publiquen en esta revista aceptan las siguientes condiciones:
- Los autores/as conservan los derechos morales de autor y ceden a la revista el derecho de la primera publicación, con el trabajo registrado con la licencia de atribución, no comercial y sin obra derivada de Creative Commons, que permite a terceros utilizar lo publicado siempre que mencionen la autoría del trabajo y a la primera publicación en esta revista, no se puede hacer uso de la obra con propósitos comerciales y no se puede utilizar las publicaciones para remezclar, transformar o crear otra obra.
- Los autores/as pueden realizar otros acuerdos contractuales independientes y adicionales para la distribución no exclusiva de la versión del artículo publicado en esta revista (p. ej., incluirlo en un repositorio institucional o publicarlo en un libro) siempre que indiquen claramente que el trabajo se publicó por primera vez en esta revista.
- Se permite y recomienda a los autores/as a publicar su trabajo en Internet (por ejemplo en páginas institucionales o personales) antes y durante el proceso de revisión y publicación, ya que puede conducir a intercambios productivos y a una mayor y más rápida difusión del trabajo publicado (vea The Effect of Open Access).