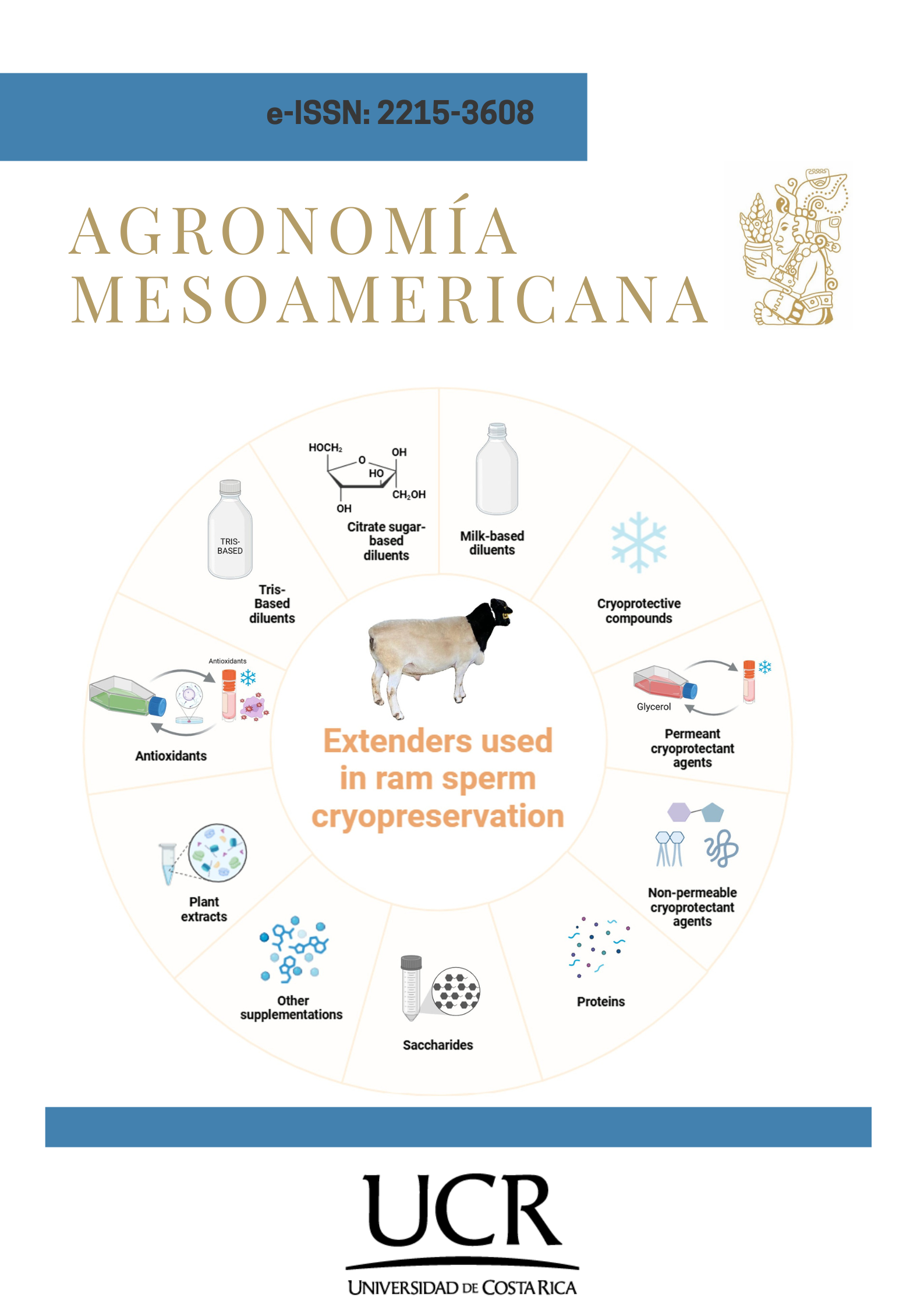
Panorama de los medios de dilución espermática para la criopreservación en carnero
DOI:
https://doi.org/10.15517/am.2024.59591Palabras clave:
congelación, diluyentes de semen, reproducción, criobiología, calidad espermáticaResumen
Introducción. Uno de los métodos más utilizados para mejorar la reproducción de los animales domésticos es la criopreservación del esperma. Para lograr los mejores resultados, este proceso complejo requiere el control meticuloso de numerosas variables. La criopreservación del esperma es una técnica utilizada en programas de mejora genética y conservación de muchas especies, especialmente los pequeños rumiantes. En este caso, el material genético de un número limitado de machos seleccionados puede utilizarse para inseminar un gran número de ovejas. Objetivo. Analizar los avances actuales en diluyentes utilizados en la criopreservación de esperma de carnero. Desarrollo. En los carneros, los espermatozoides tienen una proporción intra-membrana colesterol: fosfolípidos más baja que otras especies, lo que los hace más susceptibles a los daños asociados a la criopreservación que otros animales utilizados en la producción ganadera. La congelación y descongelación adecuada pueden evitar daños metabólicos y estructurales de los espermatozoides en la especie ovina, algo que no ha sido posible hasta el momento. El éxito del proceso de congelación de espermatozoides depende de varios factores como la velocidad de congelación/descongelación, el tiempo de equilibrio, los propios gametos o la composición del medio que rodea a los espermatozoides, entre otros. Los medios de dilución del semen podrían afectar los procesos de criopreservación. Conclusión. La vulnerabilidad del esperma de carnero al estrés criogénico plantea retos para mantener la viabilidad después de la descongelación y lograr tasas de fertilidad altas. Es necesario realizar más investigaciones centradas en refinar las estrategias de suplementación, optimizar los protocolos de congelación y explorar nuevos crioprotectores para superar estos desafíos y hacer más eficaz la criopreservación del semen de carnero para mejorar los resultados reproductivos en los programas de mejora genética ovina.
Descargas
Citas
Abdelnour, S. A., Abd El-Hack, M. E., Alagawany, M., Taha, A. E., Elnesr, S. S., Abd Elmonem, O. M., & Swelum, A. A. (2020). Useful impacts of royal jelly on reproductive sides, fertility rate and sperm traits of animals. Journal of Animal Physiology and Animal Nutrition, 104(6), 1798–1808. https://doi.org/10.1111/jpn.13303
Aisen, E. G., Medina, V. H., & Venturino, A. (2002). Cryopreservation and post-thawed fertility of ram semen frozen in different trehalose concentrations. Theriogenology, 57(7), 1801–1808. https://doi.org/10.1016/S0093-691X(02)00653-2
Aisen, E., Quintana, M., Medina, V., Morello, H., & Venturino, A. (2005). Ultramicroscopic and biochemical changes in ram spermatozoa cryopreserved with trehalose-based hypertonic extenders. Cryobiology, 50(3), 239–249. https://doi.org/10.1016/j.cryobiol.2005.02.002
Alcay, S., Berk Toker, M., Gokce, E., Ustuner, B., Tekin Onder, N., Sagirkaya, H., Nur, Z., & Kemal Soylu, M. (2015). Successful ram semen cryopreservation with lyophilized egg yolk-based extender. Cryobiology, 71(2), 329–333. https://doi.org/10.1016/j.cryobiol.2015.08.008
Allai, L., Benmoula, A., Marciane da Silva, M., Nasser, B., & El Amiri, B. (2018). Supplementation of ram semen extender to improve seminal quality and fertility rate. Animal Reproduction Science, 192, 6–17. https://doi.org/10.1016/j.anireprosci.2018.03.019
Allai, L., Druart, X., Louanjli, N., Contell, J., Nasser, B., & El Amiri, B. (2017). Improvements of ram semen quality using cactus seed oil during liquid preservation in Tris egg yolk and skim milk based extenders. Small Ruminant Research, 151, 16–21. https://doi.org/10.1016/j.smallrumres.2017.02.001
Allai, L., Druart, X., Öztürk, M., BenMoula, A., Nasser, B., & El Amiri, B. (2016). Protective effects of Opuntia ficus-indica extract on ram sperm quality, lipid peroxidation and DNA fragmentation during liquid storage. Animal Reproduction Science, 175, 1–9. https://doi.org/10.1016/j.anireprosci.2016.09.013
Allai, L., Druart, X., Terzioğlu, P., Louanjli, N., Nasser, B., Ozturk, M., & El Amiri, B. (2023). The addition of Opuntia ficus-indica ethanolic extract to a skimmed milk-based extender impacts ram sperm quality. Veterinary Medicine International, 2023(1), Article 6248890. https://doi.org/10.1155/2023/6248890
Amidi, F., Pazhohan, A., Nashtaei, M. S., Khodarahmian, M., & Nekoonam, S. (2016). The role of antioxidants in sperm freezing: a review. Cell and Tissue Banking, 17, 745–756. https://doi.org/10.1007/S10561-016-9566-5
Asadzadeh, N., Abdollahi, Z., Esmaeilkhanian, S., & Masoudi, R. (2021). Fertility and flow cytometry evaluations of ram frozen semen in plant-based extender supplemented with Mito-TEMPO. Animal Reproduction Science, 233, Article 106836. https://doi.org/10.1016/j.anireprosci.2021.106836
Barbas, J. P., Pimenta, J., Baptista, M. C., Marques, C. C., Pereira, R. M. L. N., Carolino, N., & Simões, J. (2023). Ram semen cryopreservation for Portuguese native breeds: Season and breed effects on semen quality variation. Animals, 13(4), Article 579. https://doi.org/10.3390/ani13040579
Batissaco, L., Paes de Arruda, R., Rodrigues Alves, M. B., Andrade Torres, M., Menegon Lemes, K., Romano Prado-Filho, R., Guibu de Almeida, T., De Andrade, A. F. C., & Carvalho Celeghini, E. C. (2020). Cholesterol-loaded cyclodextrin is efficient in preserving sperm quality of cryopreserved ram semen with low freezability. Reproductive Biology, 20(1), 14–24. https://doi.org/10.1016/j.repbio.2020.01.002
Ben Moula, A., Rahim, A., Chentouf, M., Raes, M., Kirschvink, N., & El Amiri, B. (2023). Inclusion of Spirulina platensis and Salvia verbenaca extracts to boost semen quality and fertilization ability in sheep. Reproduction in Domestic Animals, 58(5), 637–645. https://doi.org/10.1111/rda.14332
Benko, F., Mohammadi-Sangcheshmeh, A., Ďuračka, M., Lukáč, N., & Tvrdá, E. (2022). In vitro versus cryo-induced capacitation of bovine spermatozoa, part 1: structural, functional, and oxidative similarities and differences. PLoS ONE, 17(10), Article e0276683. https://doi.org/10.1371/journal.pone.0276683
Benson, J. D., Woods, E. J., Walters, E. M., & Critser, J. K. (2012). The cryobiology of spermatozoa. Theriogenology, 78(8), 1682–1699. https://doi.org/10.1016/j.theriogenology.2012.06.007
Bergeron, A., Brindle, Y., Blondin, P., & Manjunath, P. (2007). Milk caseins decrease the binding of the major bovine seminal plasma proteins to sperm and prevent lipid loss from the sperm membrane during sperm storage. Biology of Reproduction, 77(1), 120–126. https://doi.org/10.1095/biolreprod.106.058248
Bergeron, A., & Manjunath, P. (2006). New insights towards understanding the mechanisms of sperm protection by egg yolk and milk. Molecular Reproduction and Development, 73(10), 1338–1344. https://doi.org/10.1002/mrd.20565
Chen, Y., Meng, F., Liu, Y., Zhu, C., Ling, Y., Liu, C., Li, L., Liu, Y., He, X., Cao, J., & Zhang, Y. (2024). Effects of resveratrol on DLD and NDUFB9 decrease in frozen semen of Mongolian sheep. Cryobiology, 114, Article 104791. https://doi.org/10.1016/j.cryobiol.2023.104791
Colas, G. (1975). Effect of initial freezing temperature, addition of glycerol and dilution on the survival and fertilizing ability of deep-frozen ram semen. Reproduction, 42(2), 277–285. https://doi.org/10.1530/jrf.0.0420277
Cseh, S., Faigl, V., & Amiridis, G. S. (2012). Semen processing and artificial insemination in health management of small ruminants. Animal Reproduction Science, 130(3-4), 187–192. https://doi.org/10.1016/j.anireprosci.2012.01.014
Del Valle, I., Souter, A., Maxwell, W. M. C., Muiño-Blanco, T., & Cebrián-Pérez, J. A. (2013). Function of ram spermatozoa frozen in diluents supplemented with casein and vegetable oils. Animal Reproduction Science, 138(3-4), 213–219. https://doi.org/10.1016/j.anireprosci.2013.02.022
El-Seadawy, I. E., Kotp, M. S., El-Maaty, A. M. A., Fadl, A. M., El-Sherbiny, H. R., & Abdelnaby, E. A. (2022). The impact of varying doses of moringa leaf methanolic extract supplementation in the cryopreservation media on sperm quality, oxidants, and antioxidant capacity of frozen-thawed ram sperm. Tropical Animal Health and Production, 54(6), Article 344. https://doi.org/10.1007/s11250-022-03344-y
Emamverdi, M., Zhandi, M., Zare Shahneh, A., Sharafi, M., & Akbari-Sharif, A. (2013). Optimization of ram semen cryopreservation using a chemically defined soybean lecithin-based extender. Reproduction in Domestic Animals, 48(6), 899–904. https://doi.org/10.1111/rda.12183
Forouzanfar, M., Sharafi, M., Hosseini, S. M., Ostadhosseini, S., Hajian, M., Hosseini, L., Abedi, P., Nili, N., Rahmani, H. R., & Nasr-Esfahani, M. H. (2010). In vitro comparison of egg yolk–based and soybean lecithin–based extenders for cryopreservation of ram semen. Theriogenology, 73(4), 480–487. https://doi.org/10.1016/j.theriogenology.2009.10.005
Freitas Bittencourt, R., Oba, E., De Almeida Biscarde, C. E., Costa Azevedo, H., Vasconcelos Bittencourt, M., Oliveira de Menezes, G. F., Da Silva Lima, A., Da Mata Fuchs, K., & De Lisboa Ribeiro Filho, A. (2018). Dimethylacetamide and trehalose for ram semen cryopreservation. Cryobiology, 85, 1–6. https://doi.org/10.1016/j.cryobiol.2018.10.266
Gil, J., Lundeheim, N., Söderquist, L., & Rodrı́guez-Martı́nez, H. (2003). Influence of extender, temperature, and addition of glycerol on post-thaw sperm parameters in ram semen. Theriogenology, 59(5-6), 1241–1255. https://doi.org/10.1016/S0093-691X(02)01177-9
Gosálvez, J., López-Fernández, C., Fernández, J. L., Gouraud, A., & Holt, W. V. (2011). Relationships between the dynamics of iatrogenic DNA damage and genomic design in mammalian spermatozoa from eleven species. Molecular Reproduction and Development, 78(12), 951–961. https://doi.org/10.1002/MRD.21394
Jihad Neamah, H. (2022). Evaluation of adding prickly pear extracts to the diluted ram’s semen at preservation. Journal of Animal Health and Production, 11(1), 94–98. https://doi.org/10.17582/journal.jahp/2023/11.1.94.98
Keskin, N., Erdogan, C., Bucak, M. N., Ozturk, A. E., Bodu, M., Ili, P., Baspinar, N., & Dursun, S. (2020). Cryopreservation effects on ram sperm ultrastructure. Biopreservation and Biobanking, 18(5), 441–448. https://doi.org/10.1089/bio.2020.0056
Kumar Jha, P., Shahi Alam, M. G., Mansur, A. A., Naher, N., Islam, T., Uddin Bhuiyan, M., & Bari, F. Y. (2019). Cryopreservation of Bangladeshi ram semen using different diluents and manual freezing techniques. Cryobiology, 89, 35–41. https://doi.org/10.1016/j.cryobiol.2019.06.001
Layek, S. S., Mohanty, T. K., Kumaresan, A., & Parks, J. E. (2016). Cryopreservation of bull semen: Evolution from egg yolk based to soybean based extenders. Animal Reproduction Science, 172, 1–9. https://doi.org/10.1016/j.anireprosci.2016.04.013
Ledesma, A., Zalazar, L., Buchelly Imbachi, F., Pastore, J. I., Brown, P., Eddy, E. M., Hozbor, F., & Cesari, A. (2019). Recombinant peptide reverses cryo-capacitation in ram sperm and improves in vitro fertilization. Animal Reproduction Science, 207, 61–72. https://doi.org/10.1016/j.anireprosci.2019.05.016
Liu, G., Pan, B., Li, S., Ren, J., Wang, B., Wang, C., Su, X., & Dai, Y. (2020). Effect of bioactive peptide on ram semen cryopreservation. Cryobiology, 97, 153–158. https://doi.org/10.1016/j.cryobiol.2020.08.007
Luna-Orozco, J. R., González-Ramos, M. A., Calderón-Leyva, G., Gaytán-Alemán, L. R., Arellano-Rodríguez, F., Ángel-García, O., & Véliz-Deras, F. G. (2019). Comparison of different diluents based on liposomes and egg yolk for ram semen cooling and cryopreservation. Iranian Journal of Veterinary Research, 20(2), 126–130. https://doi.org/10.22099/ijvr.2019.5262
Marcantonini, G., Bartolini, D., Zatini, L., Costa, S., Passerini, M., Rende, M., Luca, G., Basta, G., Murdolo, G., Calafiore, R., & Galli, F. (2022). Natural cryoprotective and cytoprotective agents in cryopreservation: a focus on melatonin. Molecules, 27(10), Article 3254. https://doi.org/10.3390/molecules27103254
Masoudi, R., Sharafi, M., & Shahneh, A. Z. (2019). Effects of CoQ10 on the quality of ram sperm during cryopreservation in plant and animal based extenders. Animal Reproduction Science, 208, Article 106103. https://doi.org/10.1016/j.anireprosci.2019.06.015
Mehdipour, M., Daghigh Kia, H., Najafi, A., Vaseghi Dodaran, H., & García-Álvarez, O. (2016). Effect of green tea (Camellia sinensis) extract and pre-freezing equilibration time on the post-thawing quality of ram semen cryopreserved in a soybean lecithin-based extender. Cryobiology, 73(3), 297–303. https://doi.org/10.1016/j.cryobiol.2016.10.008
Mehdipour, M., Daghigh Kia, H., Nazari, M., & Najafi, A. (2017). Effect of lecithin nanoliposome or soybean lecithin supplemented by pomegranate extract on post-thaw flow cytometric, microscopic and oxidative parameters in ram semen. Cryobiology, 78, 34–40. https://doi.org/10.1016/j.cryobiol.2017.07.005
Moradi, M., Hajarian, H., Karamishabankareh, H., Soltani, L., & Soleymani, B. (2022). Pre-treatment of ram semen extender with magnetic nanoparticles on freeze-thawed spermatozoa. Veterinary Medicine and Science, 8(2), 792–798. https://doi.org/10.1002/vms3.689
Motlagh, M. K., Sharafi, M., Zhandi, M., Mohammadi-Sangcheshmeh, A., Shakeri, M., Soleimani, M., & Zeinoaldini, S. (2014). Antioxidant effect of rosemary (Rosmarinus officinalis L.) extract in soybean lecithin-based semen extender following freeze–thawing process of ram sperm. Cryobiology, 69(2), 217–222. https://doi.org/10.1016/j.cryobiol.2014.07.007
Murawski, M., Schwarz, T., Grygier, J., Patkowski, K., Oszczęda, Z., Jelkin, I., Kosiek, A., Gruszecki, T. M., Szymanowska, A., Skrzypek, T., Zieba, D. A., & Bartlewski, P. M. (2015). The utility of nanowater for ram semen cryopreservation. Experimental Biology and Medicine, 240(5), 611–617. https://doi.org/10.1177/1535370214557219
Najafi, A., Daghigh-Kia, H., Dodaran, H. V., Mehdipour, M., & Alvarez-Rodriguez, M. (2017). Ethylene glycol, but not DMSO, could replace glycerol inclusion in soybean lecithin-based extenders in ram sperm cryopreservation. Animal Reproduction Science, 177, 35–41. https://doi.org/10.1016/j.anireprosci.2016.12.004
Najafi, A., Najafi, M., Zanganeh, Z., Sharafi, M., Martinez-Pastor, F., & Adeldust, H. (2014). Cryopreservation of ram semen in extenders containing soybean lecithin as cryoprotectant and hyaluronic acid as antioxidant. Reproduction in Domestic Animals, 49(6), 934–940. https://doi.org/10.1111/rda.12405
Najafi, A., Zhandi, M., Towhidi, A., Sharafi, M., Akbari Sharif, A., Khodaei Motlagh, M., & Martinez-Pastor, F. (2013). Trehalose and glycerol have a dose-dependent synergistic effect on the post-thawing quality of ram semen cryopreserved in a soybean lecithin-based extender. Cryobiology, 66(3), 275–282. https://doi.org/10.1016/j.cryobiol.2013.03.002
Ofosu, J., Qazi, I. H., Fang, Y., & Zhou, G. (2021). Use of melatonin in sperm cryopreservation of farm animals: a brief review. Animal Reproduction Science, 233, Article 106850. https://doi.org/10.1016/j.anireprosci.2021.106850
Paul, R. K., Kumar, D., & Singh, R. (2021). Carboxymethyl cellulose and glycerol act synergistically as cryoprotectant during cryopreservation of ram semen. Cryobiology, 101, 61–66. https://doi.org/10.1016/j.cryobiol.2021.06.001
Peris-Frau, P., Martín-Maestro, A., Iniesta-Cuerda, M., Sánchez-Ajofrín, I., Cesari, A., Garde, J. J., Villar, M., & Soler, A. J. (2020). Cryopreservation of ram sperm alters the dynamic changes associated with in vitro capacitation. Theriogenology, 145, 100–108. https://doi.org/10.1016/j.theriogenology.2020.01.046
Pini, T., Rickard, J. P., Leahy, T., Crossett, B., Druart, X., & De Graaf, S. P. (2018). Cryopreservation and egg yolk medium alter the proteome of ram spermatozoa. Journal of Proteomics, 181, 73–82. https://doi.org/10.1016/j.jprot.2018.04.001
Plante, G., Lusignan, M.-F., Lafleur, M., & Manjunath, P. (2015). Interaction of milk proteins and binder of sperm (BSP) proteins from boar, stallion and ram semen. Reproductive Biology and Endocrinology, 13(1), Article 92. https://doi.org/10.1186/s12958-015-0093-1
Pool, K. R., Rickard, J. P., & De Graaf, S. P. (2021). Melatonin improves the motility and DNA integrity of frozen-thawed ram spermatozoa likely via suppression of mitochondrial superoxide production. Domestic Animal Endocrinology, 74, Article 106516. https://doi.org/10.1016/j.domaniend.2020.106516
Ramírez-Vasquez, R. R. A., Cano, A., Hozbor, F. A., & Cesari, A. (2019). Cryopreservation and egg yolk extender components modify the interaction between seminal plasma proteins and the sperm surface. Theriogenology, 140, 153–163. https://doi.org/10.1016/j.theriogenology.2019.08.025
Ramírez-Vasquez, R., Cesari, A., Greco, M. B., Cano, A., & Hozbor, F. (2019). Extenders modify the seminal plasma ability to minimize freeze-thaw damage on ram sperm. Reproduction in Domestic Animals, 54(12), 1621–1629. https://doi.org/10.1111/rda.13571
Riesco, M. F., Alvarez, M., Anel-Lopez, L., Neila-Montero, M., Palacin-Martinez, C., Montes-Garrido, R., Boixo, J. C., De Paz, P., & Anel, L. (2021). Multiparametric study of antioxidant effect on ram sperm cryopreservation—from field trials to research bench. Animals, 11(2), Article 283. https://doi.org/10.3390/ani11020283
Rostami, B., Ebrahimi, D., Sadeghipanah, H., Masoumi, R., & Shahir, M. H. (2020). Effects of supplementation of tris-egg yolk extender with different sugars and antioxidants on freezability of ram semen. Cryobiology, 92, 62–66. https://doi.org/10.1016/j.cryobiol.2019.10.198
Saha, A., Asaduzzaman, M., & Bari, F. Y. (2022). Cryopreservation techniques for ram sperm. Veterinary Medicine International, 2022(1), Article 7378379. https://doi.org/10.1155/2022/7378379
Salamon, S., & Maxwell, W. M. C. (2000). Storage of ram semen. Animal Reproduction Science, 62(1–3), 77–111. https://doi.org/10.1016/S0378-4320(00)00155-X
Sarıözkan, S., Bucak, M. N., Tuncer, P. B., Ulutaş, P. A., & Bilgen, A. (2009). The influence of cysteine and taurine on microscopic–oxidative stress parameters and fertilizing ability of bull semen following cryopreservation. Cryobiology, 58(2), 134–138. https://doi.org/10.1016/j.cryobiol.2008.11.006
Sharafi, M., Borghei-Rad, S. M., Hezavehei, M., Shahverdi, A., & Benson, J. D. (2022). Cryopreservation of semen in domestic animals: a review of current challenges, applications, and prospective strategies. Animals, 12(23), Article 3271. https://doi.org/10.3390/ani12233271
Silvestre, M. A., Yániz, J. L., Peña, F. J., Santolaria, P., & Castelló-Ruiz, M. (2021). Role of antioxidants in cooled liquid storage of mammal spermatozoa. Antioxidants, 10(7), Article 1096. https://doi.org/10.3390/antiox10071096
Sobeh, M., Hassan, S. A., Hassan, M. A. E., Khalil, W. A., Abdelfattah, M. A. O., Wink, M., & Yasri, A. (2020). A polyphenol-rich extract from Entada abyssinica reduces oxidative damage in cryopreserved ram semen. Frontiers in Veterinary Science, 7, Article 604477. https://doi.org/10.3389/fvets.2020.604477
Toker, M. B., Alcay, S., Gokce, E., & Ustuner, B. (2016). Cryopreservation of ram semen with antioxidant supplemented soybean lecithin-based extenders and impacts on incubation resilience. Cryobiology, 72(3), 205–209. https://doi.org/10.1016/j.cryobiol.2016.05.001
Üstüner, B., Alçay, S., Nur, Z., Sağirkaya, H., & Soylu, M. K. (2014). Effect of egg yolk and soybean lecithin on Tris-based extender in post-thaw ram semen quality and in vitro fertility. Kafkas Universitesi Veteriner Fakultesi Dergisi, 20(3), 393-398. https://scispace.com/pdf/effect-of-egg-yolk-and-soybean-lecithin-on-tris-based-1vo9a8dhsy.pdf
Valcácia Silva, S., Trindade Soares, A., Batista, A. M., Costa Almeida, F., Ferreira Nunes, J., Alves Peixoto, C., & Pessoa Guerra, M. M. (2013). Vitamin E (Trolox) addition to Tris-egg yolk extender preserves ram spermatozoon structure and kinematics after cryopreservation. Animal Reproduction Science, 137(1–2), 37–44. https://doi.org/10.1016/j.anireprosci.2012.12.002
Valente, S. S., Pereira, R. M., Baptista, M. C., Marques, C. C., Vasques, M. I., Silva Pereira, M. V. C., Horta, A. E. M., & Barbas, J. P. (2010). In vitro and in vivo fertility of ram semen cryopreserved in different extenders. Animal Reproduction Science, 117(1–2), 74–77. https://doi.org/10.1016/j.anireprosci.2009.04.007
Vieira de Souza, C., Zandonadi Brandão, F., Dantas Rodrigues Santos, J., Angélico Pereira Alfradique, V., Moreira Barbosa dos Santos, V., Da Cruz Morais, M. C., Cerqueira Rangel, P. S., Amaral da Silva, A., & Gonçalves Souza-Fabjan, J. M. (2019). Effect of different concentrations of l-carnitine in extender for semen cryopreservation in sheep. Cryobiology, 89, 104–108. https://doi.org/10.1016/j.cryobiol.2019.05.009
Watson, P. F. (1995). Recent developments and concepts in the cryopreservation of spermatozoa and the assessment of their post-thawing function. Reproduction, Fertility and Development, 7(4), 871–891. https://doi.org/10.1071/RD9950871
Yániz, J. L., Mateos, J. A., & Santolaria, P. (2012). Tris buffer improves fluorescence yield of ram spermatozoa when evaluating membrane integrity. Microscopy Research and Technique, 75(4), 520–523. https://doi.org/10.1002/jemt.21086
Zalazar, L., Iniesta-Cuerda, M., Sánchez-Ajofrín, I., Garde, J. J., Soler Valls, A. J., & Cesari, A. (2020). Recombinant SPINK3 improves ram sperm quality and in vitro fertility after cryopreservation. Theriogenology, 144, 45–55. https://doi.org/10.1016/j.theriogenology.2019.12.019
Zhang, L., Wang, X., Jiang, C., Sohail, T., Sun, Y., Sun, X., Wang, J., & Li, Y. (2024). Effects of different diluents and freezing methods on cryopreservation of Hu ram semen. Veterinary Sciences, 11(6), Article 251. https://doi.org/10.3390/vetsci11060251
Zhu, Z., Zhao, H., Cui, H., Adetunji, A. O., & Min, L. (2023). Resveratrol improves the frozen-thawed ram sperm quality. Animals, 13(24), Article 3887. https://doi.org/10.3390/ani13243887
Archivos adicionales
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2024 Francisco Sevilla, Gerald Muça, Luigj Turmalaj, Miguel A. Silvestre, Ignacio Araya, Anthony Valverde

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
1. Política propuesta para revistas de acceso abierto
Los autores/as que publiquen en esta revista aceptan las siguientes condiciones:
- Los autores/as conservan los derechos morales de autor y ceden a la revista el derecho de la primera publicación, con el trabajo registrado con la licencia de atribución, no comercial y sin obra derivada de Creative Commons, que permite a terceros utilizar lo publicado siempre que mencionen la autoría del trabajo y a la primera publicación en esta revista, no se puede hacer uso de la obra con propósitos comerciales y no se puede utilizar las publicaciones para remezclar, transformar o crear otra obra.
- Los autores/as pueden realizar otros acuerdos contractuales independientes y adicionales para la distribución no exclusiva de la versión del artículo publicado en esta revista (p. ej., incluirlo en un repositorio institucional o publicarlo en un libro) siempre que indiquen claramente que el trabajo se publicó por primera vez en esta revista.
- Se permite y recomienda a los autores/as a publicar su trabajo en Internet (por ejemplo en páginas institucionales o personales) antes y durante el proceso de revisión y publicación, ya que puede conducir a intercambios productivos y a una mayor y más rápida difusión del trabajo publicado (vea The Effect of Open Access).