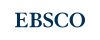Soybean leaf infusion culture media and phytotoxic metabolite production in Macrophomina phaseolina
DOI:
https://doi.org/10.15517/x1y07135Keywords:
fungal phytopathogen, secondary metabolites, bioactive molecules, charcoal rotAbstract
Introduction. The charcoal rot fungus, Macrophomina phaseolina, is a ubiquitous necrotrophic phytopathogen that infecting soybean and other plant species. Despite its significant impact on crops, limited progress has been made in understanding the factors that influence phytotoxic molecule secretion by this phytopathogen. Objective. To evaluate the effect of soybean leaf infusion in the culture medium on the differential secretion of phytotoxic molecules of M. phaseolina. Materials and methods. The study was conducted between 2016 and 2023 at the Departamento de Química Biológica, Universidad Nacional de Asunción, Paraguay. Two fungal isolates were cultured in vitro using potato dextrose broth (PDB) and Czapek-Dox broth media, with or without soybean leaf infusion. Phytotoxic activity of secreted molecules was a using soybean leaf discs. The crude organic extract from the cultures was separated using chromatographic techniques, and purified metabolites were characterized by UHPLC-PDA/MS, HRMS (APGC), HRMSESI, 1HNMR and 13CNMR. Results. Molecules secreted by M. phaseolina FCQ11 cultured in infusion-enriched PDB induced the highest percentage of necrosis. Under these conditions, three differentially secreted metabolites were isolated and identified: (R)-mellein, (3R,4R)-hydroxymellein, and (-)-botryodiplodin. Conclusions. Soybean leaf infusion presence in M. phaseolina growth media stimulates phytotoxic metabolite production and alters the profile of secreted metabolites.
Downloads
References
Abbas, H. K., Bellaloui, N., Accinelli, C., Smith, J. R., & Thomas Shier, W. (2019). Toxin production in soybean (Glycine max L.) plants with charcoal rot disease and by Macrophomina phaseolina, the fungus that causes the disease. Toxins, 11(11), Article 645. https://doi.org/10.3390/toxins11110645
Abou-Mansour, E., Débieux, J.-L., Ramírez-Suero, M., Bénard-Gellon, M., Magnin-Robert, M., Spagnolo, A., Chong, J., Farine, S., Bertsch, C., L’Haridon, F., Serrano, M., Fontaine, F., Rego, C., & Larignon, P. (2015). Phytotoxic metabolites from Neofusicoccum parvum, a pathogen of Botryosphaeria dieback of grapevine. Phytochemistry, 115, 207-215. https://doi.org/10.1016/j.phytochem.2015.01.012
Bhattacharya, G., Dhar, T. K., Bhattacharya, K., & Siddiqui, K. A. I. (1987). Mutagenic action of phaseolinone, a mycotoxin isolated from Macrophomina phaseolina. Australian Journal of Biological Sciences, 40(4), 349-354. https://doi.org/10.1071/BI9870349
Braca, A., Bader, A., & De Tommasi, N. (2012). Plant and fungi 3,4-Dihydroisocoumarins: structures, biological activity, and taxonomic relationships. In R. Atta-ur (Ed.), Studies in natural products chemistry (pp. 191-215). Elsevier. https://doi.org/10.1016/B978-0-444-59514-0.00007-9
Cabras, A., Mannoni, M. A., Serra, S., Andolfi, A., Fiore, M., & Evidente, A. (2006). Occurrence, isolation and biological activity of phytotoxic metabolites produced in vitro by Sphaeropsis sapinea, pathogenic fungus of Pinus radiata. European Journal of Plant Pathology, 115(2), 187-193. https://doi.org/10.1007/s10658-006-9006-7
Chilakala, A. R., Mali, K. V., Irulappan, V., Patil, B. S., Pandey, P., Rangappa, K., Ramegowda, V., Kumar, M. N., Puli, C. O. R., Mohan-Raju, B., & Senthil-Kumar, M. (2022). Combined drought and heat stress influences the root water relation and determine the dry root rot disease development under field conditions: a study using contrasting chickpea genotypes. Frontiers in Plant Science, 13, Article 890551. https://doi.org/10.3389/fpls.2022.890551
Chooi, Y. H., Krill, C., Barrow, R. A., Chen, S., Trengove, R., Oliver, R. P., & Solomon, P. S. (2015). An In planta-expressed polyketide synthase produces (R)-mellein in the wheat pathogen Parastagonospora nodorum. Applied and Environmental Microbiology, 81(1), 177-186. https://doi.org/10.1128/AEM.02745-14
Deshmukh, R., & Tiwari, S. (2021). Molecular interaction of charcoal rot pathogenesis in soybean: a complex interaction. Plant Cell Reports, 40, 1799-1812. https://doi.org/10.1007/s00299-021-02747-9
Dhar, T. K., Siddiqui, K. A. I., & Ali, E. (1982). Structure of phaseolinone, a novel phytotoxin from Macrophomina phaseolina. Tetrahedron Letters, 23(51), 5459-5462. https://doi.org/10.1016/0040-4039(82)80157-3
Djoukeng, J. D., Polli, S., Larignon, P., & Abou-Mansour, E. (2009). Identification of phytotoxins from Botryosphaeria obtusa, a pathogen of black dead arm disease of grapevine. European Journal of Plant Pathology, 124, 303-308. https://doi.org/10.1007/s10658-008-9419-6
Félix, C., Salvatore, M. M., DellaGreca, M., Ferreira, V., Duarte, A. S., Salvatore, F., Naviglio, D., Gallo, M., Alves, A., Esteves, A. C., & Andolfi, A. (2019). Secondary metabolites produced by grapevine strains of Lasiodiplodia theobromae grown at two different temperatures. Mycologia, 111(3), 466-476. https://doi.org/10.1080/00275514.2019.1600342
Flores-Giubi, M. E., Brito-Argáez, L., García-Sosa, K., Escalante-Erosa, F., Islas-Flores, I., & Peña-Rodríguez, L. M. (2013). Optimization of culturing conditions of a strain of Phytophthora capsici pathogenic to habanero pepper (Capsicum chinense). Journal of Phytopathology, 161(11-12), 807-813. https://doi.org/10.1111/jph.12138
Fuska, J., Nemec, P., Fuskova, A., & Kuhr, I. (1974). Antitumor antibiotics produced by Penicillium stipitatum thom. The Journal of Antibiotics, 27(2), 123-127. https://doi.org/10.7164/antibiotics.27.123
Fuska, J., Proksa, B., & Uhrín, D. (1988). The antibiotic PSX-1 Produced by Pennicilium stipitatum is identical with botryodiplodin. Folia Microbiologica, 33(3), 238-240. https://doi.org/10.1007/BF02925912
Islam, M. S., Haque, M. S., Islam, M. M., Emdad, E. M., Halim, A., Hossen, Q. M. M., Hossain, M. Z., Ahmed, B., Rahim, S., Rahman, M. S., Alam, M. M., Hou, S., Wan, X., Saito, J. A., & Alam, M. (2012). Tools to kill: Genome of one of the most destructive plant pathogenic fungi Macrophomina phaseolina. BMC Genomics, 13(1), Article 493. https://doi.org/10.1186/1471-2164-13-493
Kaur, S., Dhillon, G. S., Brar, S. K., Vallad, G. E., Chand, R., & Chauhan, V. B. (2012). Emerging phytopathogen Macrophomina phaseolina: biology, economic importance and current diagnostic trends. Critical Reviews in Microbiology, 38(2), 136-151. https://doi.org/10.3109/1040841X.2011.640977
Khambhati, V. H., Abbas, H. K., Sulyok, M., Tomaso-Peterson, M., Chen, J., & Shier, W. T. (2023). Mellein: Production in culture by Macrophomina phaseolina isolates from soybean plants exhibiting symptoms of charcoal rot and its role in pathology. Frontiers in Plant Science, 14, Article 1105590. https://doi.org/10.3389/fpls.2023.1105590
Khasin, M., Bernhardson, L. F., O’Neill, P. M., Palmer, N. A., Scully, E. D., Sattler, S. E., & Funnell-Harris, D. L. (2021). Pathogen and drought stress affect cell wall and phytohormone signaling to shape host responses in a sorghum COMT bmr12 mutant. BMC Plant Biology, 21, Article 391. https://doi.org/10.1186/s12870-021-03149-5
Li, Y., Gai, Z., Wang, C., Li, P., & Li, B. (2021). Identification of mellein as a pathogenic substance of Botryosphaeria dothidea by UPLC-MS/MS analysis and phytotoxic bioassay. Journal of Agricultural and Food Chemistry, 69(30), 8471-8481. https://doi.org/10.1021/acs.jafc.1c03249
Mahato, S. B., Siddiqui, K. A. I., Bhattacharya, G., Ghosal, T., Miyahara, K., Sholichin, M., & Kawasaki, T. (1987). Structure and stereochemistry of phaseolinic acid: a new acid from Macrophomina phaseolina. Journal of Natural Products, 50(2), 245-247. https://doi.org/10.1021/np50050a024
Marcenaro, D., & Valkonen, J. P. T. (2016). Seedborne pathogenic fungi in common bean (Phaseolus vulgaris cv. INTA rojo) in Nicaragua. PLoS ONE, 11(12), Article e0168662. https://doi.org/10.1371/journal.pone.0168662
Masi, M., Sautua, F., Zatout, R., Castaldi, S., Arrico, L., Isticato, R., Pescitelli, G., Carmona, M. A., & Evidente, A. (2021). Phaseocyclopentenones A and B, phytotoxic penta- and tetrasubstituted cyclopentenones produced by Macrophomina phaseolina, the causal agent of charcoal rot of soybean in argentina. Journal of Natural Products, 84(2), 459-465. https://doi.org/10.1021/acs.jnatprod.0c01287
Mishra, P. K., & Kumari, M. (2021). Morpho-cultural and pathogenic variability in Macrophomina phaseolina isolates from soybean. The Pharma Innovation Journal, 10(3), 777-785.
Moreau, S., Biguet, J., Lablache-Combier, A., Foulon, C., & Delfosse, M. (1982). Botryodiplodin, a mycotoxin synthesized by a strain of P. roqueforti. Journal of Organic Chemistry, 47(12), 2358-2359. https://doi.org/10.1021/jo00133a024
Nielsen, K. F., Sumarah, M. W., Frisvad, J. C., & Miller, J. D. (2006). Production of metabolites from the Penicillium roqueforti complex. Journal of Agricultural and Food Chemistry, 54(14), Article 5216. https://doi.org/10.1021/jf0680081
Nishikawa, H. (1933). Biochemistry of filamentous fungi. II: A metabolic product of Aspergillus melleus Yukawa. Part I. Bulletin of the Agricultural Chemical Society of Japan, 9(7-9), 107-109. https://doi.org/10.1080/03758397.1933.10857042
Nouri, M. T., Lawrence, D. P., Kallsen, C. E., & Trouillas, F. P. (2020). Macrophomina crown and root rot of pistachio in California. Plants, 9(2), Article 134. https://doi.org/10.3390/plants9020134
Pan, R., Bai, X., Chen, J., Zhang, H., & Wang, H. (2019). Exploring structural diversity of microbe secondary metabolites using OSMAC strategy: a literature review. Frontiers in Microbiology, 10, Article 294. https://doi.org/10.3389/fmicb.2019.00294
Pineda-Fretez, A., Orrego, A., Iehisa, J. C. M., Flores-Giubi, M. E., Barúa, J. E., Sánchez-Lucas, R., Jorrín-Novo, J., & Romero-Rodríguez, M. C. (2023). Secretome analysis of the phytopathogen Macrophomina phaseolina cultivated in liquid medium supplemented with and without soybean leaf infusion. Fungal Biology, 127(5), 1043-1052. https://doi.org/10.1016/J.FUNBIO.2023.04.001
Ramezani, M., Shier, W. T., Abbas, H. K., Tonos, J. L., Baird, R. E., & Sciumbato, G. L. (2007). Soybean charcoal rot disease fungus Macrophomina phaseolina in Mississippi produces the phytotoxin (-)-botryodiplodin but no detectable phaseolinone. Journal of Natural Products, 70(1), 128-129. https://doi.org/10.1021/np060480t
Ramírez-Suero, M., Bénard-Gellon, M., Chong, J., Laloue, H., Stempien, E., Abou-Mansour, E., Fontaine, F., Larignon, P., Mazet-Kieffer, F., Farine, S., & Bertsch, C. (2014). Extracellular compounds produced by fungi associated with Botryosphaeria dieback induce differential defence gene expression patterns and necrosis in Vitis vinifera cv. Chardonnay cells. Protoplasma, 251(6), 1417-1426. https://doi.org/10.1007/s00709-014-0643-y
Renauld, F., Moreau, S., Lablache-Combier, A., & Tiffon, B. (1985). Botryodiplodin: a mycotoxin from Penicillium roqueforti: reaction with amino-pyrimidines, amino-purines and 2’-deoxynucleosides. Tetrahedron, 41(5), 955-962. https://doi.org/10.1016/S0040-4020(01)96415-4
Reveglia, P., Masi, M., & Evidente, A. (2020). Melleins — Intriguing Natural Compounds. Biomolecules, 10(5), Article 772. https://doi.org/10.3390/biom10050772
Saeed, A. (2016). Isocoumarins, miraculous natural products blessed with diverse pharmacological activities. European Journal of Medicinal Chemistry, 116, 290-317. https://doi.org/10.1016/j.ejmech.2016.03.025
Saleh, A. A., Ahmed, H. U., Todd, T. C., Travers, S. E., Zeller, K. A., Leslie, J. F., & Garrett, K. A. (2010). Relatedness of Macrophomina phaseolina isolates from tallgrass prairie, maize, soybean and sorghum. Molecular Ecology, 19(1), 79-91. https://doi.org/10.1111/j.1365-294X.2009.04433.x
Salvatore, M. M., Félix, C., Lima, F., Ferreira, V., Naviglio, D., Salvatore, F., Duarte, A. S., Alves, A., Andolfi, A., & Esteves, A. C. (2020). Secondary metabolites produced by Macrophomina phaseolina isolated from Eucalyptus globulus. Agriculture, 10(3), Article 72. https://doi.org/10.3390/agriculture10030072
Schneider, C. A., Rasband, W. S., & Eliceiri, K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nature Methods, 9, 671-675. https://doi.org/10.1038/nmeth.2089
Slavov, S. (2005). Phytotoxins and in vitro screening for improved disease resistant plants. Biotechnology & Biotechnological Equipment, 19(Suppl. 3), 48-55. https://doi.org/10.1080/13102818.2005.10817285
Trigos, A., Reyna, S., & Matamoros, B. (1995). Macrophominol, a diketopiperazine from cultures of Macrophomina phaseolina. Phytochemistry, 40(6), 1697-1698. https://doi.org/10.1016/0031-9422(95)00626-I
Trotel-Aziz, P., Abou-Mansour, E., Courteaux, B., Rabenoelina, F., Clément, C., Fontaine, F., & Aziz, A. (2019). Bacillus subtilis PTA-271 counteracts Botryosphaeria dieback in grapevine, triggering immune responses and detoxification of fungal phytotoxins. Frontiers in Plant Science, 10, Article 25. https://doi.org/10.3389/fpls.2019.00025
Additional Files
Published
License
Copyright (c) 2025 Jazmín Vaceque-Acosta, Javier E. Barúa, Dani Daniel Ruiz-Diaz-Mendoza, M. Cristina Romero-Rodríguez, Antonio Macías-Sánchez, María Eugenia Flores-Giubi (Autor/a)

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
1. Proposed policy for open access journals
Authors who publish in this journal accept the following conditions:
a. Authors retain the copyright and assign to the journal the right to the first publication, with the work registered under the attribution, non-commercial and no-derivative license from Creative Commons, which allows third parties to use what has been published as long as they mention the authorship of the work and upon first publication in this journal, the work may not be used for commercial purposes and the publications may not be used to remix, transform or create another work.
b. Authors may enter into additional independent contractual arrangements for the non-exclusive distribution of the version of the article published in this journal (e.g., including it in an institutional repository or publishing it in a book) provided that they clearly indicate that the work was first published in this journal.
c. Authors are permitted and encouraged to publish their work on the Internet (e.g. on institutional or personal pages) before and during the review and publication process, as it may lead to productive exchanges and faster and wider dissemination of published work (see The Effect of Open Access).